Reported Adverse Effects and Attitudes among Arab Populations Following COVID-19 Vaccination: A Large-Scale Multinational Study Implementing Machine Learning Tools in Predicting Post-Vaccination Adverse Effects Based on Predisposing Factors
- PMID: 35334998
- PMCID: PMC8955470
- DOI: 10.3390/vaccines10030366
Reported Adverse Effects and Attitudes among Arab Populations Following COVID-19 Vaccination: A Large-Scale Multinational Study Implementing Machine Learning Tools in Predicting Post-Vaccination Adverse Effects Based on Predisposing Factors
Abstract
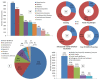
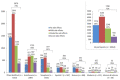
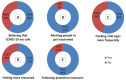
Background: The unprecedented global spread of coronavirus disease 2019 (COVID-19) has imposed huge challenges on the healthcare facilities, and impacted every aspect of life. This has led to the development of several vaccines against COVID-19 within one year. This study aimed to assess the attitudes and the side effects among Arab communities after receiving a COVID-19 vaccine and use of machine learning (ML) tools to predict post-vaccination side effects based on predisposing factors. Methods: An online-based multinational survey was carried out via social media platforms from 14 June to 31 August 2021, targeting individuals who received at least one dose of a COVID-19 vaccine from 22 Arab countries. Descriptive statistics, correlation, and chi-square tests were used to analyze the data. Moreover, extensive ML tools were utilized to predict 30 post vaccination adverse effects and their severity based on 15 predisposing factors. The importance of distinct predisposing factors in predicting particular side effects was determined using global feature importance employing gradient boost as AutoML. Results: A total of 10,064 participants from 19 Arab countries were included in this study. Around 56% were female and 59% were aged from 20 to 39 years old. A high rate of vaccine hesitancy (51%) was reported among participants. Almost 88% of the participants were vaccinated with one of three COVID-19 vaccines, including Pfizer-BioNTech (52.8%), AstraZeneca (20.7%), and Sinopharm (14.2%). About 72% of participants experienced post-vaccination side effects. This study reports statistically significant associations (p < 0.01) between various predisposing factors and post-vaccinations side effects. In terms of predicting post-vaccination side effects, gradient boost, random forest, and XGBoost outperformed other ML methods. The most important predisposing factors for predicting certain side effects (i.e., tiredness, fever, headache, injection site pain and swelling, myalgia, and sleepiness and laziness) were revealed to be the number of doses, gender, type of vaccine, age, and hesitancy to receive a COVID-19 vaccine. Conclusions: The reported side effects following COVID-19 vaccination among Arab populations are usually non-life-threatening; flu-like symptoms and injection site pain. Certain predisposing factors have greater weight and importance as input data in predicting post-vaccination side effects. Based on the most significant input data, ML can also be used to predict these side effects; people with certain predicted side effects may require additional medical attention, or possibly hospitalization.
Keywords: SARS-CoV-2; adverse reactions; coronavirus; nCoV-2019; side effects; vaccine hesitancy; vaccine safety; vaccines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Side Effects and Perceptions Following COVID-19 Vaccination in Jordan: A Randomized, Cross-Sectional Study Implementing Machine Learning for Predicting Severity of Side Effects.Vaccines (Basel). 2021 May 26;9(6):556. doi: 10.3390/vaccines9060556. Vaccines (Basel). 2021. PMID: 34073382 Free PMC article.
-
Side effects of Pfizer/BioNTech (BNT162b2) COVID-19 vaccine reported by the Birzeit University community.BMC Infect Dis. 2023 Jan 5;23(1):5. doi: 10.1186/s12879-022-07974-3. BMC Infect Dis. 2023. PMID: 36604613 Free PMC article.
-
Side Effects Associated With Homologous and Heterologous COVID-19 Vaccines: A Cross-Sectional Study in Saudi Arabia.Cureus. 2023 Jan 21;15(1):e34030. doi: 10.7759/cureus.34030. eCollection 2023 Jan. Cureus. 2023. PMID: 36814743 Free PMC article.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
-
Major severe acute respiratory coronavirus-2 (SARS-CoV-2) vaccine-associated adverse effects; benefits outweigh the risks.Expert Rev Vaccines. 2022 Oct;21(10):1377-1394. doi: 10.1080/14760584.2022.2116008. Epub 2022 Aug 24. Expert Rev Vaccines. 2022. PMID: 35986451 Review.
Cited by
-
Surveillance of Post-Vaccination Side Effects of COVID-19 Vaccines among Saudi Population: A Real-World Estimation of Safety Profile.Vaccines (Basel). 2022 Jun 10;10(6):924. doi: 10.3390/vaccines10060924. Vaccines (Basel). 2022. PMID: 35746532 Free PMC article.
-
Assessing the prevalence and patterns of COVID-19 vaccine side effects among Syrian adults: A cross-sectional study.Prev Med Rep. 2023 Dec 13;37:102558. doi: 10.1016/j.pmedr.2023.102558. eCollection 2024 Jan. Prev Med Rep. 2023. PMID: 38282667 Free PMC article.
-
Reported side-effects following Oxford/AstraZeneca COVID-19 vaccine in the north-west province, Iran: A cross-sectional study.PLoS One. 2024 Jan 5;19(1):e0296669. doi: 10.1371/journal.pone.0296669. eCollection 2024. PLoS One. 2024. PMID: 38181026 Free PMC article.
-
A First Report on Side-Effects of COVID-19 Vaccines among General Population in Sudan: A Cross-Sectional Analysis.Vaccines (Basel). 2023 Jan 31;11(2):315. doi: 10.3390/vaccines11020315. Vaccines (Basel). 2023. PMID: 36851192 Free PMC article.
-
Self-Reported Allergic Adverse Events Following Inactivated SARS-CoV-2 Vaccine (TURKOVAC™) among General and High-Risk Population.Vaccines (Basel). 2023 Feb 14;11(2):437. doi: 10.3390/vaccines11020437. Vaccines (Basel). 2023. PMID: 36851314 Free PMC article.
References
-
- Hatmal M.M., Alshaer W., Al-Hatamleh M.A.I., Hatmal M., Smadi O., Taha M.O., Oweida A.J., Boer J.C., Mohamud R., Plebanski M. Comprehensive Structural and Molecular Comparison of Spike Proteins of SARS-CoV-2, SARS-CoV and MERS-CoV, and Their Interactions with ACE2. Cells. 2020;9:2638. doi: 10.3390/cells9122638. - DOI - PMC - PubMed
-
- Al-Hatamleh M.A.I., Hatmal M.M., Sattar K., Ahmad S., Mustafa M.Z., Bittencourt M.D.C., Mohamud R. Antiviral and Immunomodulatory Effects of Phytochemicals from Honey against COVID-19: Potential Mechanisms of Action and Future Directions. Molecules. 2020;25:5017. doi: 10.3390/molecules25215017. - DOI - PMC - PubMed
-
- Al-Hatamleh M.A.I., Hatmal M.M., Alshaer W., Rahman E.N.S.E.A., Mohd-Zahid M.H., Alhaj-Qasem D.M., Yean C.Y., Alias I.Z., Jaafar J., Ferji K., et al. COVID-19 infection and nanomedicine applications for development of vaccines and therapeutics: An overview and future perspectives based on polymersomes. Eur. J. Pharm. 2021;896:173930. doi: 10.1016/j.ejphar.2021.173930. - DOI - PMC - PubMed
-
- Rzymski P., Borkowski L., Drag M., Flisiak R., Jemielity J., Krajewski J., Mastalerz-Migas A., Matyja A., Pyrc K., Simon K., et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines. 2021;9:109. doi: 10.3390/vaccines9020109. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

