Understanding the Variability of Certain Biological Properties of H1N1pdm09 Influenza Viruses
- PMID: 35335027
- PMCID: PMC8954537
- DOI: 10.3390/vaccines10030395
Understanding the Variability of Certain Biological Properties of H1N1pdm09 Influenza Viruses
Abstract
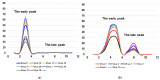
The influenza virus continually evolves because of the high mutation rate, resulting in dramatic changes in its pathogenicity and other biological properties. This study aimed to evaluate the evolution of certain essential properties, understand the connections between them, and find the molecular basis for the manifestation of these properties. To that end, 21 A(H1N1)pdm09 influenza viruses were tested for their pathogenicity and toxicity in a mouse model with a ts/non-ts phenotype manifestation and HA thermal stability. The results demonstrated that, for a strain to have high pathogenicity, it must express a toxic effect, have a non-ts phenotype, and have a thermally stable HA. The ancestor A/California/07/2009 (H1N1)pdm influenza virus expressed the non-ts phenotype, after which the cycling trend of the ts/non-ts phenotype was observed in new strains of A(H1N1)pdm09 influenza viruses, indicating that the ratio of the ts phenotype will increase in the coming years. Of the 21 tested viruses, A/South Africa/3626/2013 had the high pathogenicity in the mouse model. Sequence alignment analysis showed that this virus has three unique mutations in the polymerase complex, two of which are in the PB2 gene and one that is in the PB1 gene. Further study of these mutations might explain the distinguishing pathogenicity.
Keywords: A(H1N1)pdm09 influenza viruses; evolution; polymerase complex; temperature sensitivity of reproduction; thermal stability of the hemagglutinin; viral pathogenicity; viral toxicity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





Similar articles
-
The Effect of Mice Adaptation Process on the Pathogenicity of Influenza A/South Africa/3626/2013 (H1N1)pdm09 Model Strain.Int J Mol Sci. 2023 Dec 12;24(24):17386. doi: 10.3390/ijms242417386. Int J Mol Sci. 2023. PMID: 38139214 Free PMC article.
-
Anti-neuraminidase antibodies against pandemic A/H1N1 influenza viruses in healthy and influenza-infected individuals.PLoS One. 2018 May 9;13(5):e0196771. doi: 10.1371/journal.pone.0196771. eCollection 2018. PLoS One. 2018. PMID: 29742168 Free PMC article.
-
The temperature-sensitive and attenuation phenotypes conferred by mutations in the influenza virus PB2, PB1, and NP genes are influenced by the species of origin of the PB2 gene in reassortant viruses derived from influenza A/California/07/2009 and A/WSN/33 viruses.J Virol. 2014 Nov;88(21):12339-47. doi: 10.1128/JVI.02142-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122786 Free PMC article.
-
Segment 2 from influenza A(H1N1) 2009 pandemic viruses confers temperature-sensitive haemagglutinin yield on candidate vaccine virus growth in eggs that can be epistatically complemented by PB2 701D.J Gen Virol. 2019 Jul;100(7):1079-1092. doi: 10.1099/jgv.0.001279. Epub 2019 Jun 6. J Gen Virol. 2019. PMID: 31169484
-
Optimization of A(H1N1)pdm09 vaccine seed viruses: The source of PB1 and HA vRNA as a major determinant for antigen yield.Virus Res. 2022 Jul 2;315:198795. doi: 10.1016/j.virusres.2022.198795. Epub 2022 Apr 30. Virus Res. 2022. PMID: 35504447
Cited by
-
The Effect of Mice Adaptation Process on the Pathogenicity of Influenza A/South Africa/3626/2013 (H1N1)pdm09 Model Strain.Int J Mol Sci. 2023 Dec 12;24(24):17386. doi: 10.3390/ijms242417386. Int J Mol Sci. 2023. PMID: 38139214 Free PMC article.
-
Multi-omics data integration reveals the complexity and diversity of host factors associated with influenza virus infection.PeerJ. 2023 Oct 9;11:e16194. doi: 10.7717/peerj.16194. eCollection 2023. PeerJ. 2023. PMID: 37842064 Free PMC article.
-
Current Opinion in LAIV: A Matter of Parent Virus Choice.Int J Mol Sci. 2022 Jun 19;23(12):6815. doi: 10.3390/ijms23126815. Int J Mol Sci. 2022. PMID: 35743258 Free PMC article. Review.
-
Intradermal Immunization of Soluble Influenza HA Derived from a Lethal Virus Induces High Magnitude and Breadth of Antibody Responses and Provides Complete Protection In Vivo.Vaccines (Basel). 2023 Mar 31;11(4):780. doi: 10.3390/vaccines11040780. Vaccines (Basel). 2023. PMID: 37112692 Free PMC article.
References
-
- WHO Seasonal Ifluenza. [(accessed on 1 February 2022)]. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenz....
-
- WHO Pandemic Influenza Risk Management Interim Guidance. [(accessed on 1 February 2022)]. Available online: https://www.icao.int/APAC/Meetings/2014%20CAPSCAAP7/Pandemic%20Influenza....
-
- WHO Pandemic Influenza. [(accessed on 1 February 2022)]. Available online: https://www.euro.who.int/en/health-topics/communicable-diseases/influenz....
LinkOut - more resources
Full Text Sources
Miscellaneous

