HLA Class II Polymorphism and Humoral Immunity Induced by the SARS-CoV-2 mRNA-1273 Vaccine
- PMID: 35335034
- PMCID: PMC8949280
- DOI: 10.3390/vaccines10030402
HLA Class II Polymorphism and Humoral Immunity Induced by the SARS-CoV-2 mRNA-1273 Vaccine
Abstract
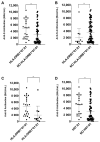
The vaccines designed against the SARS-CoV-2 coronavirus are based on the spike (S) protein. Processing of the S protein by antigen-presenting cells (APC) and its subsequent presentation to T cells is an essential part of the development of a humoral response. HLA-class II alleles are considered immune response genes because their codified molecules, expressed on the surface of APCs (macrophages, dendritic, and B cells) present antigenic peptides to T cell via their T cell receptor (TCR). The HLA-class II genes are highly polymorphic, regulating what specific peptides induce follicular helper T cells (TFH) and promote B lymphocyte differentiation into plasma or memory B cells. This work hypothesizes that the presence of certain HLA-class II alleles could be associated with the intensity of the humoral response (amount, length) to the SARS-CoV2 mRNA 1273 vaccine. We have studied the relationship between the HLA-class II typing of 87 health workers and the level of antibodies produced 30 days after vaccination. We show a possible association between the HLA-DRB1* 07:01 allele and the HLA-DRB1*07:01~DQA1*02:01~DQB1*02:02 haplotype to a higher production of antibodies 30 days after the administration of the second dose of mRNA-1273.
Keywords: HLA associations; anti-S antibodies; mRNA-1273 vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

