A Comparative Analysis of COVID-19 Vaccines Based on over 580,000 Cases from the Vaccination Adverse Event Reporting System
- PMID: 35335040
- PMCID: PMC8950485
- DOI: 10.3390/vaccines10030408
A Comparative Analysis of COVID-19 Vaccines Based on over 580,000 Cases from the Vaccination Adverse Event Reporting System
Abstract
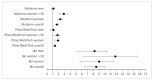
Background: The COVID-19 pandemic is being battled via the largest vaccination campaign in history, with more than eight billion doses administered thus far. Therefore, discussions about potentially adverse reactions, and broader safety concerns, are critical. The U.S. Vaccination Adverse Event Reporting System (VAERS) has recorded vaccination side effects for over 30 years. About 580,000 events have been filed for COVID-19 thus far, primarily for the Johnson & Johnson (New Jersey, USA), Pfizer/BioNTech (Mainz, Germany), and Moderna (Cambridge, USA) vaccines. Methods: Using available databases, we evaluated these three vaccines in terms of the occurrence of four generally-noticed adverse reactions—namely, cerebral venous sinus thrombosis, Guillain−Barré syndrome (a severe paralytic neuropathy), myocarditis, and pericarditis. Our statistical analysis also included a calculation of odds ratios (ORs) based on total vaccination numbers, accounting for incidence rates in the general population. Results: ORs for a number of adverse events and patient groups were (largely) increased, most notably for the occurrence of cerebral venous sinus thrombosis after vaccination with the Johnson & Johnson vaccine. The overall population OR of 10 increases to 12.5 when limited to women, and further yet (to 14.4) among women below age 50 yrs. In addition, elevated risks were found (i) for Guillain−Barré syndrome (OR of 11.6) and (ii) for myocarditis/pericarditis (ORs of 5.3/4.1, respectively) among young men (<25 yrs) vaccinated with the Pfizer/BioNTech vaccine. Conclusions: Any conclusions from such a retrospective, real-world data analysis must be drawn cautiously, and should be confirmed by prospective double-blinded clinical trials. In addition, we emphasize that the adverse events reported here are not specific side effects of COVID vaccines, and the significant, well-established benefits of COVID-19 vaccination outweigh the potential complications surveyed here.
Keywords: COVID-19; Guillain–Barré syndrome; carditis; real-world evidence; thrombosis; vaccine safety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Moderna Clinical Study Protocol. [(accessed on 24 November 2021)]. Available online: https://www.modernatx.com/sites/default/files/mRNA-1273-P301-Protocol.pdf.
-
- Updated Recommendations from the Advisory Committee on Immunization Practices for Use of the Janssen (Johnson & Johnson) COVID-19 Vaccine After Reports of Thrombosis with Thrombocytopenia Syndrome Among Vaccine Recipients—United States, April 2021. [(accessed on 24 November 2021)]; Available online: https://www.cdc.gov/mmwr/volumes/70/wr/mm7017e4.htm. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

