Waning Humoral Response after COVID-19 mRNA Vaccination in Maintenance Dialysis Patients and Recovery after a Complementary Third Dose
- PMID: 35335065
- PMCID: PMC8950255
- DOI: 10.3390/vaccines10030433
Waning Humoral Response after COVID-19 mRNA Vaccination in Maintenance Dialysis Patients and Recovery after a Complementary Third Dose
Abstract
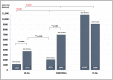
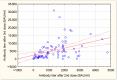
The aim of this study was to analyze the waning of anti-spike (S) antibodies after mRNA vaccination against COVID-19 in maintenance dialysis patients, and to assess the safety and effectiveness of the complementary third dose. This was a prospective, longitudinal study in which we analyzed the kinetics of antibodies up to six months after a two-dose vaccination (first protocol) in infection-naïve dialysis patients (IN-Ds), previously infected dialysis patients (PI-Ds) and subjects without chronic kidney disease (the controls), as well as their humoral response to the third dose of the same mRNA vaccine (second protocol). The respective reduction in antibody titer after 3 and 6 months by 82.9% and 93.03% in IN-Ds (n = 109), 73.4% and 93.36% in PI-Ds (n = 32) and 75.5% and 88.8% in the controls (n = 20) was demonstrated. Consequently, a protective antibody titer above 141 BAU/mL was found in only 47.7% and 23.8% of IN-Ds after 3 and 6 months, respectively. After the third vaccine dose, a significant increase in antibody titer was observed in all groups, with increases by a factor of ×51.6 in IN-Ds, ×30.1 in the controls and ×8.4 in PI-Ds. The median antibody titer after the third dose differed significantly between groups, and was the highest in PI-Ds: PI-Ds, 9090 (3300−15,000) BAU/mL; the controls, 6945 (2130−11,800); IN-Ds, 3715 (1470−7325) (p < 0.001). In conclusion, we observed similar degrees of antibody waning in all patients. After 3 months, over half of the infection-naïve dialysis patients had a very low antibody titer, and almost twenty percent of them had no antibodies at all. The humoral response to the third dose was very good, raising their titer of antibodies to a higher level than those in the general population who have received the primary two-dose scheme. The results support the administration of a complementary third dose of the mRNA vaccine for dialysis patients as soon as possible.
Keywords: COVID-19; SARS-CoV-2; humoral immunity; mRNA vaccines; maintenance dialysis patients; seroconversion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dickerman B.A., Gerlovin H., Madenci A.L., Kurgansky K.E., Ferolito B.R., Figueroa Muñiz M.J., Gagnon D.R., Gaziano J.M., Cho K., Casas J.P., et al. Comparative effectiveness of BNT162b2 and mRNA-1273 Vaccines in U.S. Veterans. N. Engl. J. Med. 2021;386:105–115. doi: 10.1056/NEJMoa2115463. - DOI - PMC - PubMed
-
- Barda N., Dagan N., Cohen C., Hernan M.A., Lipsitch M., Kohane I.S., Reis B.Y., Balicer R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet. 2021;398:P2093–P2100. doi: 10.1016/S0140-6736(21)02249-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

