SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine
- PMID: 35335079
- PMCID: PMC8950166
- DOI: 10.3390/vaccines10030448
SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine
Abstract
Background: At variance to humoral responses, cellular immunity after anti-SARS-CoV-2 vaccines has been poorly explored in recipients of allogeneic hematopoietic stem-cell transplantation (Allo-HSCT), especially within the first post-transplant years where immunosuppression is more profound and harmful.
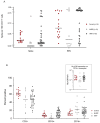
Methods: SARS-CoV-2 Spike protein-specific T-cell responses were explored after two doses of BNT162b2 mRNA vaccine in 45 Allo-HSCT recipients with a median time from transplant of less than 2 years by using INF-γ ELISPOT assay and flow-cytometry enumeration of CD4+ and CD8+ T lymphocytes with intracellular cytokine production of IFN-γ and TNF-α.
Results: A strong TNF-α+ response from SARS-CoV-2-specific CD4+ T-cells was detected in a majority of humoral responders (89%) as well as in a consistent population of non-humoral responders (40%).
Conclusions: T-cells are likely to participate in protection against COVID-19 viral infection, even in the absence of detectable antibody response, especially in the first years post-transplant in Allo-HSCT recipients.
Keywords: BNT162b2; CD4+ T cells; CD8+ T cells; COVID 19; IFNγ; SARS-CoV-2 mRNA; TNFα; allogeneic hematopoietic stem cell transplantation; cellular immunity; humoral immunity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest, except Patrice Chevallier who received honoraria from Pfizer outside the submitted work.
Figures


References
-
- Barrière J., Chamorey E., Adjtoutah Z., Castelnau O., Mahamat A., Marco S., Petit E., Leysalle A., Raimondi V., Carles M. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann. Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

