Fever after Vaccination against SARS-CoV-2 with mRNA-Based Vaccine Associated with Higher Antibody Levels during 6 Months Follow-Up
- PMID: 35335080
- PMCID: PMC8950492
- DOI: 10.3390/vaccines10030447
Fever after Vaccination against SARS-CoV-2 with mRNA-Based Vaccine Associated with Higher Antibody Levels during 6 Months Follow-Up
Abstract
Background: The effect of post-vaccination adverse events on immunogenicity is unknown. We aimed to explore relationship between post-vaccination adverse reactions and antibody levels during 6-month follow-up.
Methods: Blood was serially drawn from healthcare workers after the second dose of BNT162b2 mRNA vaccine (Day 12, 30, 60, 90, 120, 150, and 180) and anti-SARS-CoV-2 spike IgG (S-IgG) levels were measured. Following each vaccine dose, volunteers completed a questionnaire regarding adverse reactions (symptomatic vs. asymptomatic groups).
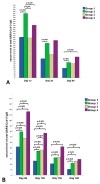
Results: A total of 395 subjects received the second dose of the vaccine. The main results were as follows: (i) fever after the 2nd dose was independently associated with the median S-IgG level at all follow-up time points; (ii) significantly higher S-IgG levels were observed in the symptomatic group of patients without prior COVID-19 infection throughout the entire follow-up period; (iii) prior COVID-19 positivity resulted in higher S-IgG levels only in the asymptomatic group from Day 90 of the follow-up period; (iv) both prior COVID-19 disease with asymptomatic status and symptomatic status without prior COVID-19 infection resulted in similar S-IgG antibody levels; (v) significantly lower serum S-IgG levels were observed in smokers.
Conclusion: Fever may play an important role in the post-vaccination immune response in the long term.
Keywords: COVID-19; adverse reaction; anti-SARS-CoV-2; fever; mRNA vaccine; spike IgG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- [(accessed on 29 January 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
-
- [(accessed on 25 January 2022)]. Available online: https://ourworldindata.org/COVID-vaccinations.
LinkOut - more resources
Full Text Sources
Miscellaneous

