Systemic Adverse Effects Induced by the BNT162b2 Vaccine Are Associated with Higher Antibody Titers from 3 to 6 Months after Vaccination
- PMID: 35335084
- PMCID: PMC8950942
- DOI: 10.3390/vaccines10030451
Systemic Adverse Effects Induced by the BNT162b2 Vaccine Are Associated with Higher Antibody Titers from 3 to 6 Months after Vaccination
Abstract
Objective: We aimed to determine the relationship between vaccine-related adverse effects and antibody (Ab) titers from 3 to 6 months after the second dose of the BNT162b2 coronavirus disease 2019 (COVID-19) mRNA vaccine (Pfizer/BioNTech) in Japan.
Methods: We enrolled 378 healthcare workers (255 women and 123 men) whose Ab titers were analyzed 3 and 6 months after the second dose in our previous study and whose characteristics and adverse effects were collected previously by using a structured self-report questionnaire.
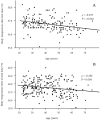
Results: The workers' median age was 44 years. Although injection-site symptoms occurred with almost equal frequency between the first and second doses, systemic adverse effects, such as general fatigue and fever, were significantly more frequent after the second dose than after the first dose. Multivariate analysis showed that fever was significantly correlated with female participants for the second dose (odds ratio (OR), 2.139; 95% confidence interval (95% CI), 1.185-3.859), older age for the first dose (OR, 0.962; 95% CI, 0.931-0.994) and second dose (OR, 0.957; 95% CI, 0.936-0.979), and dyslipidemia for the first dose (OR, 8.750; 95% CI, 1.814-42.20). Age-adjusted Ab titers at 3 months after vaccination were 23.7% and 23.4% higher in patients with a fever than in those without a fever after the first and second dose, respectively. In addition, age-adjusted Ab titers at 3 and 6 months after the second dose were, respectively, 21.7% and 19.3% higher in the group in which an anti-inflammatory agent was used than in the group without the use of an anti-inflammatory agent.
Conclusion: Participants with systemic adverse effects tend to have higher Ab titers from 3 to 6 months after the second dose of the BNT162b2 vaccine. Our results may encourage vaccination, even among people with vaccine hesitancy related to relatively common systemic adverse effects.
Keywords: SARS-CoV-2; adverse effect; clinical epidemiology; viral infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Kozakai R., Kushida A., Moumouni P.F., Okuma S., Takahashi K., Hoshi K., Sato Y., Takahashi M., Chida N., Takahashi M., et al. Assessment of COVID-19 mRNA vaccination titer and side effects in healthy volunteers. J. Lab. Med. 2021 doi: 10.1515/labmed-2021-0156. - DOI
-
- Izumo T., Kuse N., Awano N., Tone M., Sakamoto K., Takada K., Muto Y., Fujimoto K., Saiki A., Ito Y., et al. Side effects and antibody titer transition of the BNT162b2 messenger ribonucleic acid coronavirus disease 2019 vaccine in Japan. Respir. Investig. 2021;59:635–642. doi: 10.1016/j.resinv.2021.06.003. - DOI - PMC - PubMed
-
- Coggins S.A., Laing E.D., Olsen C.H., Goguet E., Moser M., Jackson-Thompson B.M., Samuels E.C., Pollett S.D., Tribble D.R., Davies J., et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. Open Forum Infect. Dis. 2021;9:ofab575. doi: 10.1093/ofid/ofab575. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

