Genetic Modification of T Cells for the Immunotherapy of Cancer
- PMID: 35335089
- PMCID: PMC8949949
- DOI: 10.3390/vaccines10030457
Genetic Modification of T Cells for the Immunotherapy of Cancer
Abstract
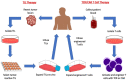
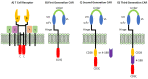
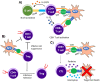
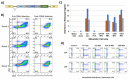
Immunotherapy is a beneficial treatment approach for multiple cancers, however, current therapies are effective only in a small subset of patients. Adoptive cell transfer (ACT) is a facet of immunotherapy where T cells targeting the tumor cells are transferred to the patient with several primary forms, utilizing unmodified or modified T cells: tumor-infiltrating lymphocytes (TIL), genetically modified T cell receptor transduced T cells, and chimeric antigen receptor (CAR) transduced T cells. Many clinical trials are underway investigating the efficacy and safety of these different subsets of ACT, as well as trials that combine one of these subsets with another type of immunotherapy. The main challenges existing with ACT are improving clinical responses and decreasing adverse events. Current research focuses on identifying novel tumor targeting T cell receptors, improving safety and efficacy, and investigating ACT in combination with other immunotherapies.
Keywords: adoptive cell transfer; cancer immunotherapy; chimeric antigen receptors; gene-modified TCR transduced T cells; tumor-infiltrating lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Allison J.P., McIntyre B.W., Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J. Immunol. 1982;129:2293–2300. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

