Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models
- PMID: 35335093
- PMCID: PMC8949948
- DOI: 10.3390/vaccines10030461
Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models
Abstract
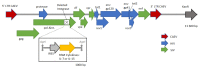
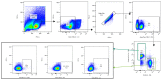
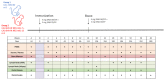
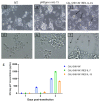
HIV-1 remains a major public health issue worldwide in spite of efficacious antiviral therapies, but with no cure or preventive vaccine. The latter has been very challenging, as virus infection is associated with numerous escape mechanisms from host specific immunity and the correlates of protection remain incompletely understood. We have developed an innovative vaccine strategy, inspired by the efficacy of live-attenuated virus, but with the safety of a DNA vaccine, to confer both cellular and humoral responses. The CAL-SHIV-IN- lentiDNA vaccine comprises the backbone of the pathogenic SHIVKU2 genome, able to mimic the early phase of viral infection, but with a deleted integrase gene to ensure safety precluding integration within the host genome. This vaccine prototype, constitutively expressing viral antigen under the CAEV LTR promoter, elicited a variety of vaccine-specific, persistent CD4 and CD8 T cells against SIV-Gag and Nef up to 80 weeks post-immunization in cynomolgus macaques. Furthermore, these specific responses led to antiviral control of the pathogenic SIVmac251. To further improve the efficacy of this vaccine, we incorporated the IL-7 or IL-15 genes into the CAL-SHIV-IN- plasmid DNA in efforts to increase the pool of vaccine-specific memory T cells. In this study, we examined the immunogenicity of the two co-injected lentiDNA vaccines CAL-SHIV-IN- IRES IL-7 and CAL-SHIV-IN- IRES IL-15 in BALB/cJ mice and rhesus macaques and compared the immune responses with those generated by the parental vaccine CAL-SHIV-IN-. This co-immunization elicited potent vaccine-specific CD4 and CD8 T cells both in mice and rhesus macaques. Antibody-dependent cell-mediated cytotoxicity (ADCC) antibodies were detected up to 40 weeks post-immunization in both plasma and mucosal compartments of rhesus macaques and were enhanced by the cytokines.
Keywords: CAEV LTR; DNA vaccine; HIV-1; IL-15; IL-7; LentiDNA; Rhesus macaques; SHIV.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Immunogenicity of a lentiviral-based DNA vaccine driven by the 5'LTR of the naturally attenuated caprine arthritis encephalitis virus (CAEV) in mice and macaques.Vaccine. 2012 Apr 19;30(19):2956-62. doi: 10.1016/j.vaccine.2012.02.050. Epub 2012 Mar 2. Vaccine. 2012. PMID: 22387218 Free PMC article.
-
Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques.Vaccine. 2007 Jun 21;25(26):4967-82. doi: 10.1016/j.vaccine.2006.11.070. Epub 2007 Feb 14. Vaccine. 2007. PMID: 17335943
-
Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologous SHIVs and simian immunodeficiency virus.Virology. 2002 Sep 30;301(2):189-205. doi: 10.1006/viro.2002.1544. Virology. 2002. PMID: 12359422
-
A single lentivector DNA based immunization contains a late heterologous SIVmac251 mucosal challenge infection.Vaccine. 2020 May 6;38(21):3729-3739. doi: 10.1016/j.vaccine.2020.03.053. Epub 2020 Apr 9. Vaccine. 2020. PMID: 32278522
-
New prospects for the development of a vaccine against human immunodeficiency virus type 1. An overview.C R Acad Sci III. 1999 Nov;322(11):959-66. doi: 10.1016/s0764-4469(00)87193-0. C R Acad Sci III. 1999. PMID: 10646090 Review.
Cited by
-
SHIV-C109p5 NHP induces rapid disease progression in elderly macaques with extensive GI viral replication.J Virol. 2024 Feb 20;98(2):e0165223. doi: 10.1128/jvi.01652-23. Epub 2024 Feb 1. J Virol. 2024. PMID: 38299866 Free PMC article.
-
Optimization of In Vivo Electroporation Conditions and Delivery of DNA Vaccine Encoding SARS-CoV-2 RBD Using the Determined Protocol.Pharmaceutics. 2022 Oct 22;14(11):2259. doi: 10.3390/pharmaceutics14112259. Pharmaceutics. 2022. PMID: 36365078 Free PMC article.
-
Genetic adjuvants: A paradigm shift in vaccine development and immune modulation.Mol Ther Nucleic Acids. 2025 Apr 8;36(2):102536. doi: 10.1016/j.omtn.2025.102536. eCollection 2025 Jun 10. Mol Ther Nucleic Acids. 2025. PMID: 40336572 Free PMC article. Review.
-
Progress and Recent Developments in HIV Vaccine Research.Vaccines (Basel). 2025 Jun 26;13(7):690. doi: 10.3390/vaccines13070690. Vaccines (Basel). 2025. PMID: 40733667 Free PMC article. Review.
-
Enhanced HIV immune responses elicited by an apoptotic single-cycle SHIV lentivector DNA vaccine.Front Cell Infect Microbiol. 2025 Apr 10;15:1481427. doi: 10.3389/fcimb.2025.1481427. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40292218 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

