The Antagonizing Role of Heme in the Antimalarial Function of Artemisinin: Elevating Intracellular Free Heme Negatively Impacts Artemisinin Activity in Plasmodium falciparum
- PMID: 35335120
- PMCID: PMC8949904
- DOI: 10.3390/molecules27061755
The Antagonizing Role of Heme in the Antimalarial Function of Artemisinin: Elevating Intracellular Free Heme Negatively Impacts Artemisinin Activity in Plasmodium falciparum
Abstract
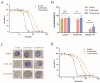
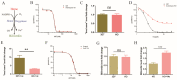
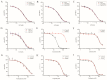
The rich source of heme within malarial parasites has been considered to underly the action specificity of artemisinin. We reasoned that increasing intraparasitic free heme levels might further sensitize the parasites to artemisinin. Various means, such as modulating heme synthesis, degradation, polymerization, or hemoglobin digestion, were tried to boost intracellular heme levels, and under several scenarios, free heme levels were significantly augmented. Interestingly, all results arrived at the same conclusion, i.e., elevating heme acted in a strongly negative way, impacting the antimalarial action of artemisinin, but exerted no effect on several other antimalarial drugs. Suppression of the elevated free heme level by introducing heme oxygenase expression effectively restored artemisinin potency. Consistently, zinc protoporphyrin IX/zinc mesoporphyrin, as analogues of heme, drastically increased free heme levels and, concomitantly, the EC50 values of artemisinin. We were unable to effectively mitigate free heme levels, possibly due to an unknown compensating heme uptake pathway, as evidenced by our observation of efficient uptake of a fluorescent heme homologue by the parasite. Our results thus indicate the existence of an effective and mutually compensating heme homeostasis network in the parasites, including an uncharacterized heme uptake pathway, to maintain a certain level of free heme and that augmentation of the free heme level negatively impacts the antimalarial action of artemisinin. Importance: It is commonly believed that heme is critical in activating the antimalarial action of artemisinins. In this work, we show that elevating free heme levels in the malarial parasites surprisingly negatively impacts the action of artemisinin. We tried to boost free heme levels with various means, such as by modulating heme synthesis, heme polymerization, hemoglobin degradation and using heme analogues. Whenever we saw elevation of free heme levels, reduction in artemisinin potency was also observed. The homeostasis of heme appears to be complex, as there exists an unidentified heme uptake pathway in the parasites, nullifying our attempts to effectively reduce intraparasitic free heme levels. Our results thus indicate that too much heme is not good for the antimalarial action of artemisinins. This research can help us better understand the biological properties of this mysterious drug.
Keywords: artemisinins; heme; heme oxygenase; hemozoin; triarylimidazole 14c.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- WHO . World Malaria Report 2018. WHO; Geneva, Switzerland: 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

