Apigenin Isolated from Carduus crispus Protects against H2O2-Induced Oxidative Damage and Spermatogenic Expression Changes in GC-2spd Sperm Cells
- PMID: 35335140
- PMCID: PMC8955133
- DOI: 10.3390/molecules27061777
Apigenin Isolated from Carduus crispus Protects against H2O2-Induced Oxidative Damage and Spermatogenic Expression Changes in GC-2spd Sperm Cells
Abstract
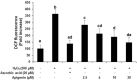
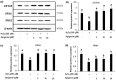
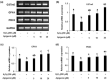
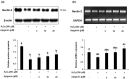
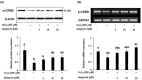
Testicular oxidative stress is one of the most common factors underlying male infertility. Welted thistle, Carduus crispus Linn., and its bioactive principles are attracting scientific interest in treating male reproductive dysfunctions. Here, the protective effects of apigenin isolated from C. crispus against oxidative damage induced by hydrogen peroxide (H2O2) and dysregulation in spermatogenesis associated parameters in testicular sperm cells was investigated. Cell viabilities, ROS scavenging effects, and spermatogenic associated molecular expressions were measured by MTT, DCF-DA, Western blotting and real-time RT-PCR, respectively. A single peak with 100% purity of apigenin was obtained in HPLC conditions. Apigenin treated alone (2.5, 5, 10 and 20 µM) did not exhibit cytotoxicity, but inhibited the H2O2-induced cellular damage and elevated ROS levels significantly (p < 0.05 at 5, 10 and 20 µM) and dose-dependently. Further, H2O2-induced down-regulation of antioxidant (glutathione S-transferases m5, glutathione peroxidase 4, and peroxiredoxin 3) and spermatogenesis-associated (nectin-2 and phosphorylated-cAMP response element-binding protein) molecular expression in GC-2spd cells were attenuated by apigenin at both protein and mRNA levels (p < 0.05). In conclusion, our study showed that apigenin isolated from C. crispus might be an effective agent that can protect ROS-induced testicular dysfunctions.
Keywords: CREB; apigenin; hydrogen peroxide; nectin; reactive oxygen species; testicular cells; welted thistle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fatima S. Novel Prospects in Oxidative and Nitrosative Stress. InTechOpen; London, UK: 2018. Role of Reactive Oxygen Species in Male Reproduction.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

