Microwave-Assisted Solution Synthesis of Metastable Intergrowth of AgInS2 Polymorphs
- PMID: 35335179
- PMCID: PMC8954084
- DOI: 10.3390/molecules27061815
Microwave-Assisted Solution Synthesis of Metastable Intergrowth of AgInS2 Polymorphs
Abstract
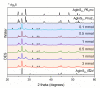
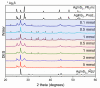
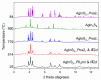
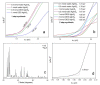
The intergrowth of stable and metastable AgInS2 polymorphs was synthesized using a microwave-assisted synthesis. The samples were synthesized in water and in a deep eutectic solvent (DES) consisting of choline chloride and thiourea. An increase in the metal precursor concentration improved the crystallinity of the synthesized samples and affected the particle size. AgInS2 cannot be synthesized from crystalline binary Ag2S or In2S3 via this route. The solution synthesis reported here results in the intergrowth of the thermodynamically stable polymorph (space group I4¯2d, chalcopyrite structure) and the high-temperature polymorph (space group Pna21, wurtzite-like structure) that is metastable at room temperature. A scanning transmission microscopy (STEM) study revealed the intergrowth of tetragonal and orthorhombic polymorphs in a single particle and unambiguously established that the long-thought hexagonal wurtzite polymorph has pseudo-hexagonal symmetry and is best described with the orthorhombic unit cell. The solution-synthesized AgInS2 polymorphs intergrowth has slightly lower bandgap values in the range of 1.73 eV-1.91 eV compared to the previously reported values for tetragonal I4¯2d (1.86 eV) and orthorhombic Pna21 (1.98 eV) polymorphs.
Keywords: STEM; deep eutectic solvent (DES); green synthesis; metastable; morphology; semiconductor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Abbott A.P., Capper G., Davies D.L., McKenzie K.J., Obi S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data. 2006;51:1280–1282. doi: 10.1021/je060038c. - DOI
-
- Hong S.K., Doughty R.M., Osterloh F.E., Zaikina J.V. Deep eutectic solvent route synthesis of zinc and copper vanadate n-type semiconductors-mapping oxygen vacancies and their effect on photovoltage. J. Mater. Chem. A. 2019;7:12303–12316. doi: 10.1039/C9TA00957D. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

