Preparation and Synthetic Applications of Five-to-Seven-Membered Cyclic α-Diazo Monocarbonyl Compounds
- PMID: 35335391
- PMCID: PMC8954351
- DOI: 10.3390/molecules27062030
Preparation and Synthetic Applications of Five-to-Seven-Membered Cyclic α-Diazo Monocarbonyl Compounds
Abstract
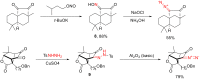
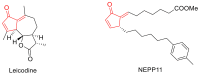
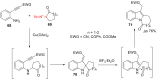
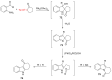
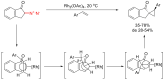
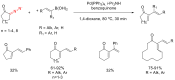
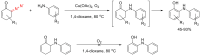
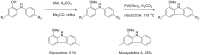
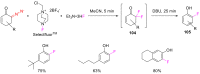
The reactivity of cyclic α-diazo monocarbonyl compounds differs from that of their acyclic counterparts. In this review, we summarize the current literature available on the synthesis and synthetic applications of three major classes of cyclic α-diazo monocarbonyl compounds: α-diazo ketones, α-diazo lactones and α-diazo lactams.
Keywords: preparation; synthetic applications; α-diazo ketones; α-diazo lactams; α-diazo lactones.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




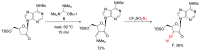



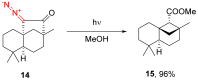

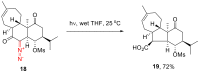





















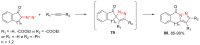








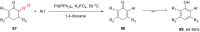


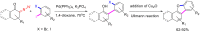



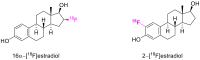








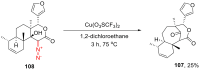
























Similar articles
-
Rhodium-catalyzed rearrangement of alpha-diazo thiol esters to thio-substituted ketenes. Application in the synthesis of cyclobutanones, cyclobutenones, and beta-lactams.J Org Chem. 2000 Jul 14;65(14):4375-84. doi: 10.1021/jo000227c. J Org Chem. 2000. PMID: 10891141
-
Catalytic Asymmetric Homologation of Ketones with α-Alkyl α-Diazo Esters.J Am Chem Soc. 2021 Feb 10;143(5):2394-2402. doi: 10.1021/jacs.0c12683. Epub 2021 Jan 28. J Am Chem Soc. 2021. PMID: 33507075
-
Homologation Reaction of Ketones with Diazo Compounds.Chem Rev. 2016 Mar 9;116(5):2937-81. doi: 10.1021/acs.chemrev.5b00381. Epub 2016 Feb 8. Chem Rev. 2016. PMID: 26854865 Review.
-
Novel N-transfer reagent for converting α-amino acid derivatives to α-diazo compounds.Chem Commun (Camb). 2021 May 14;57(39):4839-4842. doi: 10.1039/d1cc01285a. Epub 2021 Apr 19. Chem Commun (Camb). 2021. PMID: 33870368
-
λ5-Phosphorus-Containing α-Diazo Compounds: A Valuable Tool for Accessing Phosphorus-Functionalized Molecules.Chem Rev. 2016 Nov 23;116(22):13991-14055. doi: 10.1021/acs.chemrev.6b00373. Epub 2016 Oct 24. Chem Rev. 2016. PMID: 27775327 Review.
References
-
- Ye T., McKervey M.A. Organic Synthesis with a-Diazo Carbonyl Compounds. Chem. Rev. 1994;94:1091–1160. doi: 10.1021/cr00028a010. - DOI
-
- Burke S.D., Grieco P.A. Organic Reactions. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 1979. Intramolecular Reactions of Diazocarbonyl Compounds; pp. 361–475.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

