Soft Colloidal Particles at Fluid Interfaces
- PMID: 35335463
- PMCID: PMC8956102
- DOI: 10.3390/polym14061133
Soft Colloidal Particles at Fluid Interfaces
Abstract
The assembly of soft colloidal particles at fluid interfaces is reviewed in the present paper, with emphasis on the particular case of microgels formed by cross-linked polymer networks. The dual polymer/colloid character as well as the stimulus responsiveness of microgel particles pose a challenge in their experimental characterization and theoretical description when adsorbed to fluid interfaces. This has led to a controversial and, in some cases, contradictory picture that cannot be rationalized by considering microgels as simple colloids. Therefore, it is necessary to take into consideration the microgel polymer/colloid duality for a physically reliable description of the behavior of the microgel-laden interface. In fact, different aspects related to the above-mentioned duality control the organization of microgels at the fluid interface, and the properties and responsiveness of the obtained microgel-laden interfaces. This works present a critical revision of different physicochemical aspects involving the behavior of individual microgels confined at fluid interfaces, as well as the collective behaviors emerging in dense microgel assemblies.
Keywords: deformability; interfaces; interfacial tension; microgels; reconfigurable; softness.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



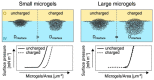



References
-
- Maestro A., Guzman E. Colloids at Fluid Interfaces. Processes. 2019;7:942. doi: 10.3390/pr7120942. - DOI
-
- Maestro A., Santini E., Zabiegaj D., Llamas S., Ravera F., Liggieri L., Ortega F., Rubio R.G., Guzman E. Particle and Particle-Surfactant Mixtures at Fluid Interfaces: Assembly, Morphology, and Rheological Description. Adv. Condens. Matter Phys. 2015;2015:917516. doi: 10.1155/2015/917516. - DOI
-
- Maestro A., Guzman E., Ortega F., Rubio R.G. Contact angle of micro- and nanoparticles at fluid interfaces. Curr. Opin. Colloid Interface Sci. 2014;19:355–367. doi: 10.1016/j.cocis.2014.04.008. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

