Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing
- PMID: 35335540
- PMCID: PMC8951233
- DOI: 10.3390/polym14061207
Employing Cellulose Nanofiber-Based Hydrogels for Burn Dressing
Abstract
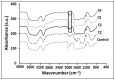
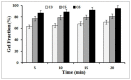
The aim of this research was to fabricate a burn dressing in the form of hydrogel films constructed with cellulose nanofibers (CNF) that has pain-relieving properties, in addition to wound healing. In this study, the hydrogels were prepared in the form of film. For this, CNF at weight ratios of 1, 2, and 3 wt.%, 1 wt.% of hydroxyethyl cellulose (HEC), and citric acid (CA) crosslinker with 10 and 20 wt.% were used. FE-SEM analysis showed that the structure of the CNF was preserved after hydrogel preparation. Cationization of CNF by C6H14NOCl was confirmed by FTIR spectroscopy. The drug release analysis results showed a linear relationship between the amount of absorption and the concentration of the drug. The MTT test (assay protocol for cell viability and proliferation) showed the high effectiveness of cationization of CNF and confirmed the non-toxicity of the resulting hydrogels.
Keywords: burn dressing; cellulose nanofibers; cross-linker; hydrogel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

