Pigment Produced by Glycine-Stimulated Macrophomina Phaseolina Is a (-)-Botryodiplodin Reaction Product and the Basis for an In-Culture Assay for (-)-Botryodiplodin Production
- PMID: 35335604
- PMCID: PMC8951085
- DOI: 10.3390/pathogens11030280
Pigment Produced by Glycine-Stimulated Macrophomina Phaseolina Is a (-)-Botryodiplodin Reaction Product and the Basis for an In-Culture Assay for (-)-Botryodiplodin Production
Abstract
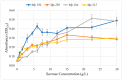
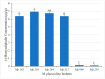
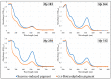
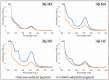
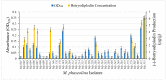
An isolate of Macrophomina phaseolina from muskmelons (Cucumis melo)was reported by Dunlap and Bruton to produce red pigment(s) in melons and in culture in the presence of added glycine, alanine, leucine, or asparagine in the medium, but not with some other amino acids and nitrogen-containing compounds. We explored the generality and mechanism of this pigment production response using pathogenic M. phaseolina isolates from soybean plants expressing symptoms of charcoal rot disease. A survey of 42 M. phaseolina isolates growing on Czapek-Dox agar medium supplemented with glycine confirmed pigment production by 71% of isolates at the optimal glycine concentration (10 g/L). Studies in this laboratory have demonstrated that some pathogenic isolates of M. phaseolina produce the mycotoxin (-)-botryodiplodin, which has been reported to react with amino acids, proteins, and other amines to produce red pigments. Time course studies showed a significant positive correlation between pigment and (-)-botryodiplodin production by selected M. phaseolina isolates with maximum production at seven to eight days. Pigments produced in agar culture medium supplemented with glycine, beta-alanine, or other amines exhibited similar UV-vis adsorption spectra as did pigments produced by (±)-botryodiplodin reacting in the same agar medium. In a separate study of 39 M. phaseolina isolates, red pigment production (OD520) on 10 g/L glycine-supplemented Czapek-Dox agar medium correlated significantly with (-)-botryodiplodin production (LC/MS analysis of culture filtrates) in parallel cultures on un-supplemented medium. These results support pigment production on glycine-supplemented agar medium as a simple and inexpensive in-culture method for detecting (-)-botryodiplodin production by M. phaseolina isolates.
Keywords: (−)-Botryodiplodin; Macrophomina phaseolina; amino acid; charcoal rot disease; in-culture bioassay; kojic acid; moniliformin; mycotoxin; natural product; phytotoxin; pigments; secondary metabolite; soybean.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schmidt O. Wood and Tree Fungi. Springer; Berlin/Heidelberg, Germany: 2006. p. 125.
-
- Dunlap J.R., Bruton D.B. Pigment biosynthesis by Macrophomina phaseolina: The glycine-specific requirement. Trans. Br. Mycol. Soc. 1986;86:111–115. doi: 10.1016/S0007-1536(86)80122-X. - DOI
-
- Dhingra O.D., Sinclair J.B. Location of Macrophomina phaseolina on soybean plants related to culture characteristics and virulence. Phytopathology. 1973;63:934–936. doi: 10.1094/Phyto-63-934. - DOI
-
- Ghosh T., Biswas M.K., Guin C., Roy P. A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol. Mol. Biol. 2018;19:72–84.
-
- Mengistu A., Ray J.D., Smith J.R., Paris R.L. Charcoal rot disease assessment of soybean genotypes using a colony-forming unit index. Crop Sci. 2007;47:2453–2461. doi: 10.2135/cropsci2007.04.0186. - DOI
LinkOut - more resources
Full Text Sources

