Sexual Dimorphism of the Neuroimmunoendocrine Response in the Spleen during a Helminth Infection: A New Role for an Old Player?
- PMID: 35335632
- PMCID: PMC8955289
- DOI: 10.3390/pathogens11030308
Sexual Dimorphism of the Neuroimmunoendocrine Response in the Spleen during a Helminth Infection: A New Role for an Old Player?
Abstract
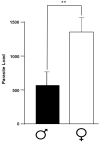
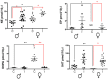
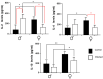
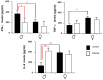
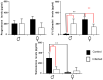
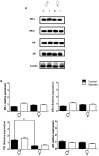
The interaction of the nervous, immune, and endocrine systems is crucial in maintaining homeostasis in vertebrates, and vital in mammals. The spleen is a key organ that regulates the neuroimmunoendocrine system. The Taenia crassiceps mouse system is an excellent experimental model to study the complex host-parasite relationship, particularly sex-associated susceptibility to infection. The present study aimed to determine the changes in neurotransmitters, cytokines, sex steroids, and sex-steroid receptors in the spleen of cysticercus-infected male and female mice and whole parasite counts. We found that parasite load was higher in females in comparison to male mice. The levels of the neurotransmitter epinephrine were significantly decreased in infected male animals. The expression of IL-2 and IL-4 in the spleen was markedly increased in infected mice; however, the expression of Interleukin (IL)-10 and interferon (IFN)-γ decreased. We also observed sex-associated differences between non-infected and infected mice. Interestingly, the data show that estradiol levels increased in infected males but decreased in females. Our studies provide evidence that infection leads to changes in neuroimmunoendocrine molecules in the spleen, and these changes are dimorphic and impact the establishment, growth, and reproduction of T. crassiceps. Our findings support the critical role of the neuroimmunoendocrine network in determining sex-associated susceptibility to the helminth parasite.
Keywords: Helminths; Taenia crassiceps; cysticercosis; cytokines; immunity; infection; neuroimmunoendocrinology; neurotransmitters; parasite immunity; sexual dimorphism; spleen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Larralde C., Sciutto E., Huerta L., Terrazas I., Fragoso G., Trueba L., Lemus D., Lomelí C., Tapia G., Montoya R.M. Experimental cysticercosis by Taenia crassiceps in mice: Factors involved in susceptibility. Acta Leiden. 1989;57:131–134. - PubMed
-
- Larralde C., Sotelo J., Montoya R.M., Palencia G., Padilla A., Govezensky T., Diaz M.L., Sciutto E. Immunodiagnosis of human cysticercosis in cerebrospinal fluid. Antigens from murine Taenia crassiceps cysticerci effectively substitute those from porcine Taenia solium. Arch. Pathol. Lab. Med. 1990;114:926–928. - PubMed
-
- Larralde C., Laclette J.P., Montoya R.M., Contreras L., Sandoval M., Bojalil R., Owen C.S., Arzate J., Goodsaid F., Diaz M.L., et al. Reliable Serology of Taenia solium Cysticercosis with Antigens from Cyst Vesicular Fluid: Elisa and Hemagglutination Tests. Am. J. Trop. Med. Hyg. 1986;35:965–973. doi: 10.4269/ajtmh.1986.35.965. - DOI - PubMed
-
- Toledo A., Fragoso G., Rosas G., Hernández M., Gevorkian G., López-Casillas F., Hernández B., Acero G., Huerta M., Larralde C., et al. Two Epitopes Shared by Taenia crassiceps and Taenia solium Confer Protection against Murine T. crassiceps Cysticercosis along with a Prominent T1 Response. Infect. Immun. 2001;69:1766–1773. doi: 10.1128/IAI.69.3.1766-1773.2001. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

