The Role of Nuclear Factor Kappa B (NF-κB) in the Immune Response against Parasites
- PMID: 35335634
- PMCID: PMC8950322
- DOI: 10.3390/pathogens11030310
The Role of Nuclear Factor Kappa B (NF-κB) in the Immune Response against Parasites
Abstract
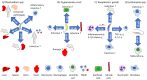
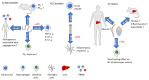
The immune system consists of various cells, organs, and processes that interact in a sophisticated manner to defend against pathogens. Upon initial exposure to an invader, nonspecific mechanisms are raised through the activation of macrophages, monocytes, basophils, mast cells, eosinophils, innate lymphoid cells, or natural killer cells. During the course of an infection, more specific responses develop (adaptive immune responses) whose hallmarks include the expansion of B and T cells that specifically recognize foreign antigens. Cell to cell communication takes place through physical interactions as well as through the release of mediators (cytokines, chemokines) that modify cell activity and control and regulate the immune response. One regulator of cell states is the transcription factor Nuclear Factor kappa B (NF-κB) which mediates responses to various stimuli and is involved in a variety of processes (cell cycle, development, apoptosis, carcinogenesis, innate and adaptive immune responses). It consists of two protein classes with NF-κB1 (p105/50) and NF-κB2 (p100/52) belonging to class I, and RelA (p65), RelB and c-Rel belonging to class II. The active transcription factor consists of a dimer, usually comprised of both class I and class II proteins conjugated to Inhibitor of κB (IκB). Through various stimuli, IκB is phosphorylated and detached, allowing dimer migration to the nucleus and binding of DNA. NF-κB is crucial in regulating the immune response and maintaining a balance between suppression, effective response, and immunopathologies. Parasites are a diverse group of organisms comprised of three major groups: protozoa, helminths, and ectoparasites. Each group induces distinct effector immune mechanisms and is susceptible to different types of immune responses (Th1, Th2, Th17). This review describes the role of NF-κB and its activity during parasite infections and its contribution to inducing protective responses or immunopathologies.
Keywords: NF-κB; RelA; immune response; p50; p65; parasites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Berasain P., Carmona C., Frangione B., Dalton J.P., Goñi F. Fasciola hepatica: Parasite-secreted proteinases degrade all human IgG subclasses: Determination of the specific cleavage sites and identification of the immunoglobulin fragments produced. Exp. Parasitol. 2000;94:99–110. doi: 10.1006/expr.1999.4479. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

