Drug Sensitivity of Vaccine-Derived Rubella Viruses and Quasispecies Evolution in Granulomatous Lesions of Two Ataxia-Telangiectasia Patients Treated with Nitazoxanide
- PMID: 35335662
- PMCID: PMC8955873
- DOI: 10.3390/pathogens11030338
Drug Sensitivity of Vaccine-Derived Rubella Viruses and Quasispecies Evolution in Granulomatous Lesions of Two Ataxia-Telangiectasia Patients Treated with Nitazoxanide
Abstract
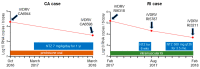
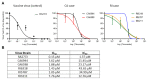
A strong association between rubella virus (RuV) and chronic granulomas, in individuals with inborn errors of immunity, has been recently established. Both the RA27/3 vaccine and wild-type RuV strains were highly sensitive to a broad-spectrum antiviral drug, nitazoxanide (NTZ), in vitro. However, NTZ treatment, used as a salvage therapy, resulted in little or no improvements of RuV-associated cutaneous granulomas in patients. Here, we report investigations of possible causes of treatment failures in two ataxia-telangiectasia patients. Although a reduction in RuV RNA in skin lesions was detected by real-time RT-PCR, live immunodeficiency-related vaccine-derived rubella viruses (iVDRV) were recovered from granulomas, before and after the treatments. Tizoxanide, an active NTZ metabolite, inhibited replications of all iVDRVs in cultured A549 cells, but the 50% and 90% inhibitory concentrations were 10-40 times higher than those for the RA27/3 strain. There were no substantial differences in iVDRV sensitivities, neither before nor after treatments. Analysis of quasispecies in the E1 gene, a suspected NTZ target, showed no effect of NTZ treatments on quasispecies' complexity in lesions. Thus, failures of NTZ therapies were likely due to low sensitivities of iVDRVs to the drug, and not related to the emergence of resistance, following long-term NTZ treatments.
Keywords: ataxia-telangiectasia; cutaneous granulomas; immunodeficiency-related vaccine-derived rubella viruses (iVDRV); nitazoxanide; quasispecies.
Conflict of interest statement
The authors declare no conflict of interest. (The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention).
Figures

References
-
- Hobman T. Rubella virus. In: David M., Knipe P.H., editors. Fields Virology 1. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 687–711.
-
- Plotkin S., Reef S., Cooper L., Alford C.A. Rubella. In: Remington J., Klein J., Wilson C., Nizet V., Maldonato Y., editors. Infectious Diseases of the Fetus and Newborn Infant. Elsveier; Philadelphia, PA, USA: 2011. pp. 861–898.
-
- McLean H.Q., Fiebelkorn A.P., Temte J.L., Wallace G.S. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: Summary recommendations of the Advisory Committee on Immunization Practices (ACIP) Morb. Mortal. Wkly. Rep. Recomm. Rep. 2013;62:1–34. - PubMed
-
- Rubin L.G., Levin M.J., Ljungman P., Davies E.G., Avery R.K., Tomblyn M., Bousvaros A., Dhanireddy S., Sung L., Keyserling H., et al. Executive Summary: 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host. Clin. Infect. Dis. 2014;58:309–318. doi: 10.1093/cid/cit816. - DOI - PubMed

