Ex Vivo Infection of Human Placental Explants by Trypanosoma cruzi Reveals a microRNA Profile Similar to That Seen in Trophoblast Differentiation
- PMID: 35335686
- PMCID: PMC8952303
- DOI: 10.3390/pathogens11030361
Ex Vivo Infection of Human Placental Explants by Trypanosoma cruzi Reveals a microRNA Profile Similar to That Seen in Trophoblast Differentiation
Abstract
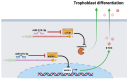
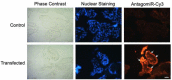
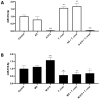
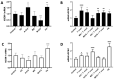
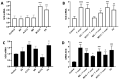
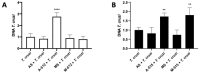
Congenital Chagas disease, caused by the protozoan parasite Trypanosoma cruzi, is responsible for 22.5% of new cases each year. However, placental transmission occurs in only 5% of infected mothers and it has been proposed that the epithelial turnover of the trophoblast can be considered a local placental defense against the parasite. Thus, Trypanosoma cruzi induces cellular proliferation, differentiation, and apoptotic cell death in the trophoblast, which are regulated, among other mechanisms, by small non-coding RNAs such as microRNAs. On the other hand, ex vivo infection of human placental explants induces a specific microRNA profile that includes microRNAs related to trophoblast differentiation such as miR-512-3p miR-515-5p, codified at the chromosome 19 microRNA cluster. Here we determined the expression validated target genes of miR-512-3p and miR-515-5p, specifically human glial cells missing 1 transcription factor and cellular FLICE-like inhibitory protein, as well as the expression of the main trophoblast differentiation marker human chorionic gonadotrophin during ex vivo infection of human placental explants, and examined how the inhibition or overexpression of both microRNAs affects parasite infection. We conclude that Trypanosoma cruzi-induced trophoblast epithelial turnover, particularly trophoblast differentiation, is at least partially mediated by placenta-specific miR-512-3p and miR-515-5p and that both miRNAs mediate placental susceptibility to ex vivo infection of human placental explants. Knowledge about the role of parasite-modulated microRNAs in the placenta might enable their use as biomarkers, as prognostic and therapeutic tools for congenital Chagas disease in the future.
Keywords: Trypanosoma cruzi; miRNAs; placenta; trophoblast differentiation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
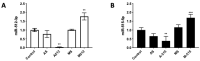
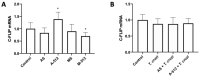
References
-
- Lenk E.J., Redekop W.K., Luyendijk M., Fitzpatrick C., Niessen L., Stolk W.A., Tediosi F., Rijnsburger A.J., Bakker R., Hontelez J.A.C., et al. Socioeconomic Benefit to Individuals of Achieving 2020 Targets for Four Neglected Tropical Diseases Controlled/Eliminated by Innovative and Intensified Disease Management: Human African Trypanosomiasis, Leprosy, Visceral Leishmaniasis, Chagas Disease. PLOS Negl. Trop. Dis. 2018;12:e0006250. doi: 10.1371/journal.pntd.0006250. - DOI - PMC - PubMed
-
- Kemmerling U., Osuna A., Schijman A.G.A.G., Truyens C. Congenital Transmission of Trypanosoma cruzi: A Review About the Interactions Between the Parasite, the Placenta, the Maternal and the Fetal/Neonatal Immune Responses. Front. Microbiol. 2019;10:1854. doi: 10.3389/fmicb.2019.01854. - DOI - PMC - PubMed
-
- WHO Chagas Disease (American Trypanosomiasis) [(accessed on 12 December 2021)]. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1.
-
- WHO Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates. Relev. Epidemiol. Hebd. 2015;90:33–43. - PubMed
-
- Picado A., Cruz I., Redard-Jacot M., Schijman A.G., Torrico F., Sosa-Estani S., Katz Z., Ndung’u J.M., Ndung’u J.M. The Burden of Congenital Chagas Disease and Implementation of Molecular Diagnostic Tools in Latin America. BMJ Glob. Health. 2018;3:e001069. doi: 10.1136/bmjgh-2018-001069. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

