Influence of the Micro-Nanostructuring of Titanium Dioxide Films on the Photocatalytic Degradation of Formic Acid under UV Illumination
- PMID: 35335821
- PMCID: PMC8953088
- DOI: 10.3390/nano12061008
Influence of the Micro-Nanostructuring of Titanium Dioxide Films on the Photocatalytic Degradation of Formic Acid under UV Illumination
Abstract
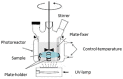
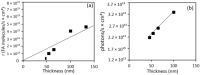
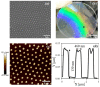
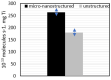
Surface micro-nanostructuring can provide new functionalities and properties to coatings. For example, it can improve the absorption efficiency, hydrophobicity and/or tribology properties. In this context, we studied the influence of micro-nanostructuring on the photocatalytic efficiency of sol-gel TiO2 coatings during formic acid degradation under UV illumination. The micro-nanostructuring was performed using the UV illumination of microspheres deposited on a photopatternable sol-gel layer, leading to a hexagonal arrangement of micropillars after development. The structures and coatings were characterized using Raman spectroscopy, ellipsometry, atomic force microscopy and scanning electron microscopy. When the sol-gel TiO2 films were unstructured and untreated at 500 °C, their effect on formic acid's degradation under UV light was negligible. However, when the films were annealed at 500 °C, they crystallized in the anatase phase and affected the degradation of formic acid under UV light, also depending on the thickness of the layer. Finally, we demonstrated that surface micro-nanostructuring in the form of nanopillars can significantly increase the photocatalytic efficiency of a coating during the degradation of formic acid under UV light.
Keywords: formic acid; micro-nanostructuring; photocatalysis; sol-gel.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures




References
-
- Li X., Sun X. Interface Design and Development of Coating Materials in Lithium–Sulfur Batteries. Adv. Funct. Mater. 2018;28:1801323. doi: 10.1002/adfm.201801323. - DOI
-
- Hołyńska M., Tighe A., Semprimoschnig C. Coatings and Thin Films for Spacecraft Thermo-Optical and Related Functional Applications. Adv. Mater. Interfaces. 2018;5:1701644. doi: 10.1002/admi.201701644. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

