pH-Responsive Nanoparticles for Delivery of Paclitaxel to the Injury Site for Inhibiting Vascular Restenosis
- PMID: 35335910
- PMCID: PMC8949492
- DOI: 10.3390/pharmaceutics14030535
pH-Responsive Nanoparticles for Delivery of Paclitaxel to the Injury Site for Inhibiting Vascular Restenosis
Abstract
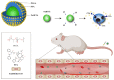
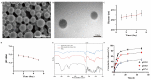
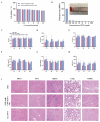
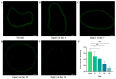
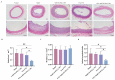
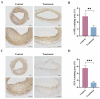
A high incidence of restenosis has been reported at the site of inflammation following angioplasty and stent implantation. The anti-proliferative drug paclitaxel (PTX) could help to reduce inflammation and restenosis; however, it has poor water solubility and serious adverse side effects at high doses. Given the presence of metabolic acidosis at the site of inflammation, we hypothesized that nanoparticles that are responsive to low pH could precisely release the loaded drug at the target site. We successfully constructed pH-responsive poly(D, L-lactic-co-glycolic acid) (PLGA) nanoparticles loaded with PTX and NaHCO3 as a pH-sensitive therapeutic agent (PTX-NaHCO3-PLGA NPs). The NPs exhibited remarkable pH sensitivity and a good safety profile both in vitro in rat vascular smooth muscle cells and in vivo in Sprague Dawley rats after tail vein injection. In the rat model, the PTX-NaHCO3-PLGA NPs treatment group showed suppressed intimal proliferation following balloon-induced carotid artery injury compared with that of the saline-treated control. Overall, these results demonstrate that our newly developed pH-responsive nanodrug delivery platform has the potential to effectively inhibit restenosis.
Keywords: drug delivery; pH-responsive nanoparticles; paclitaxel; sodium bicarbonate; vascular restenosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Synthesis, characterization, and evaluation of paclitaxel loaded in six-arm star-shaped poly(lactic-co-glycolic acid).Int J Nanomedicine. 2013;8:4315-26. doi: 10.2147/IJN.S51629. Epub 2013 Nov 7. Int J Nanomedicine. 2013. PMID: 24235829 Free PMC article.
-
A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases.Biomaterials. 2020 Feb;230:119605. doi: 10.1016/j.biomaterials.2019.119605. Epub 2019 Nov 8. Biomaterials. 2020. PMID: 31740099
-
Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis.Biomaterials. 2016 Oct;105:167-184. doi: 10.1016/j.biomaterials.2016.08.003. Epub 2016 Aug 4. Biomaterials. 2016. PMID: 27522252
-
The prevention of restenosis in vivo with a VEGF gene and paclitaxel co-eluting stent.Biomaterials. 2013 Feb;34(6):1635-43. doi: 10.1016/j.biomaterials.2012.11.006. Epub 2012 Nov 28. Biomaterials. 2013. PMID: 23199742
-
Drug-eluting stents.Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review.
Cited by
-
Nanoparticle-based approaches for treating restenosis after vascular injury.Front Pharmacol. 2024 Oct 24;15:1427651. doi: 10.3389/fphar.2024.1427651. eCollection 2024. Front Pharmacol. 2024. PMID: 39512830 Free PMC article. Review.
-
Curcumin/pEGCG-encapsulated nanoparticles enhance spinal cord injury recovery by regulating CD74 to alleviate oxidative stress and inflammation.J Nanobiotechnology. 2024 Oct 24;22(1):653. doi: 10.1186/s12951-024-02916-4. J Nanobiotechnology. 2024. PMID: 39443923 Free PMC article.
-
Mechanism of action and potential therapeutic targets of TGF-β-related signaling pathway and its downstream miRNA expression in pulmonary arterial hypertension.Front Pharmacol. 2025 Jun 6;16:1596767. doi: 10.3389/fphar.2025.1596767. eCollection 2025. Front Pharmacol. 2025. PMID: 40548051 Free PMC article. Review.
-
Chitosan-salvianolic acid B coating on the surface of nickel-titanium alloy inhibits proliferation of smooth muscle cells and promote endothelialization.Front Bioeng Biotechnol. 2023 Nov 13;11:1300336. doi: 10.3389/fbioe.2023.1300336. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38026871 Free PMC article.
-
Use of stimulatory responsive soft nanoparticles for intracellular drug delivery.Nano Res. 2023;16(5):6974-6990. doi: 10.1007/s12274-022-5267-5. Epub 2023 Jan 14. Nano Res. 2023. PMID: 36685637 Free PMC article. Review.
References
-
- Axel D.I., Kunert W., Goggelmann C., Oberhoff M., Herdeg C., Kuttner A., Wild D.H., Brehm B.R., Riessen R., Koveker G., et al. Paclitaxel Inhibits Arterial Smooth Muscle Cell Proliferation and Migration In Vitro and In Vivo Using Local Drug Delivery. Circulation. 1997;96:636–645. doi: 10.1161/01.CIR.96.2.636. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

