Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection
- PMID: 35335932
- PMCID: PMC8955626
- DOI: 10.3390/pharmaceutics14030556
Three Birds, One Excipient: Development of an Improved pH, Isotonic, and Buffered Ketamine Formulation for Subcutaneous Injection
Abstract
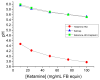
Subcutaneous (SC) ketamine has been found to be effective in pain management, though reports of injection site irritation and sterile abscesses exist with currently available ketamine HCl formulations. Such adverse SC reactions are commonly associated with low pH, high osmolality and/or high injection volumes. An optimal SC formulation of ketamine would thus have a pH and osmolality close to physiological levels, without compromising on concentration and, thus, injection volume. Such a formulation should also be buffered to maintain the pH at the acceptable level for extended time periods. As many of these physicochemical properties are interrelated, achieving these aims represented a significant challenge in formulation development. We describe the development of a novel Captisol®-based formulation strategy to achieve an elevated pH, isosmotic and buffered formulation of ketamine (hence, three birds, one excipient) without compromising on concentration. This strategy has the potential to be readily adapted to other amine-based APIs.
Keywords: captisol; cyclodextrin; ketamine; ketamine formulation; osmolality; subcutaneous injection.
Conflict of interest statement
J.W. is a consultant and owns shares in Bexson Biomedical Inc. T.E. is a consultant and owns shares in Bexson Biomedical, Inc. J.B. is cofounder and Chief Science Officer of Bexson Biomedical, Inc. J.W. and J.B. are inventors on patent: U.S. Patent No. 10,973,780.
Figures



References
-
- Del Valle E.M.M. Cyclodextrins and Their Uses: A Review. Process Biochem. 2004;39:1033–1046. doi: 10.1016/S0032-9592(03)00258-9. - DOI
-
- [(accessed on 28 December 2021)]. Available online: https://www.captisol.com/technology/how-it-works#:~:text=Captisol%C2%AE%....
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

