Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa
- PMID: 35335946
- PMCID: PMC8955998
- DOI: 10.3390/pharmaceutics14030570
Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa
Abstract
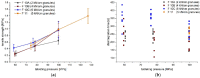
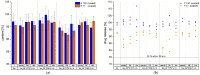
Epidermolysis bullosa is a genetically heterogenous skin fragility disorder with multiorgan involvement appearing already in newborn children. Severe progressive fibrosis follows skin blistering, mucosa lesions, and wound healing, favouring development of highly aggressive squamous cell carcinomas. Losartan potassium (LP) has been described to show positive effects; therefore, it was of clinical interest to develop 2 mm mini-tablets with LP for treatment of the affected children. Several challenges emerged during development: limited flowability and sticking to punches were observed in the first tableting experiments due to a high drug load, and a bitter taste of the LP was reported. Sticking to punches was reduced by using SMCC 50 and a combination of different lubricants; however, direct compression trials on a Korsch XM 12 rotary press were not successful due to compaction phenomena in the hopper. Thus, an intermediate dry granulation was successfully introduced. Two final formulations of the mini-tablets complied with the requirements of the European Pharmacopoeia regarding disintegration times (<15 min) and friability (<1.0%); mean tensile strengths amounted to about 1 MPa as a compromise between manufacturability and sufficient mechanical strength for further coating studies. The subsequent coating step succeeded delaying the initial drug release for more than 2 min. An acceptance value ≤15 was matched for the coated mini-tablets, and stability studies showed a promising shelf life.
Keywords: coating; dry granulation; epidermolysis bullosa; losartan potassium; mini-tablet; paediatrics; rare disease; stability.
Conflict of interest statement
Luis Canha is an employee of Midas Pharma.
Figures






References
-
- European Parliament and Council REGULATION (EC) No 1901/2006 (12 December 2006) on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. 2006. [(accessed on 8 December 2021)]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1901....
-
- WHO Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children. 2008. [(accessed on 6 December 2021)]. Available online: https://www.who.int/selection_medicines/committees/expert/17/application....
LinkOut - more resources
Full Text Sources
Research Materials

