Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives
- PMID: 35335969
- PMCID: PMC8950151
- DOI: 10.3390/pharmaceutics14030593
Synthesis and Evaluation of the Antifungal and Toxicological Activity of Nitrofuran Derivatives
Abstract
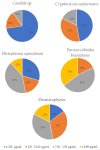
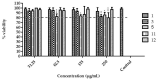
Fungal diseases affect more than 1 billion people worldwide. The constant global changes, the advent of new pandemics, and chronic diseases favor the diffusion of fungal pathogens such as Candida, Cryptococcus, Aspergillus, Trichophyton, Histoplasma capsulatum, and Paracoccidioides brasiliensis. In this work, a series of nitrofuran derivatives were synthesized and tested against different fungal species; most of them showed inhibitory activity, fungicide, and fungistatic profile. The minimal inhibitory concentration (MIC90) values for the most potent compounds range from 0.48 µg/mL against H. capsulatum (compound 11) and P. brasiliensis (compounds 3 and 9) to 0.98 µg/mL against Trichophyton rubrum and T. mentagrophytes (compounds 8, 9, 12, 13 and 8, 12, 13, respectively), and 3.9 µg/mL against Candida and Cryptococcus neoformans strains (compounds 1 and 5, respectively). In addition, all compounds showed low toxicity when tested in vitro on lung cell lines (A549 and MRC-5) and in vivo in Caenorhabditis elegans larvae. Many of them showed high selectivity index values. Thus, these studied nitrofuran derivatives proved to be potent against different fungal species, characterized by low toxicity and high selectivity; for these reasons, they may become promising compounds for the treatment of mycoses.
Keywords: Caenorhabditis elegans larvae; Candida sp.; Cryptococcus neoformans; Histoplasma capsulatum; Paracoccidioides brasiliensis; Trichophyton mentagrophytes; Trichophyton rubrum; antifungal activity; broad-spectrum antifungal; nitrofuran derivates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
- 2020/15586-4 (COV)/Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP
- 2019/22188-8 (NMB)/Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP
- 2017/18388-6 (CBC-O)/Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP
- 16/11836-0 (MJSM-G)/Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP
- 001; 88887.500765/2020-00 (COV)/Programa de Apoio ao Desenvolvimento Científico (PADC) da Faculdade de Ciências Farmacêuticas da UNESP, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
LinkOut - more resources
Full Text Sources

