Liposomal siRNA Formulations for the Treatment of Herpes Simplex Virus-1: In Vitro Characterization of Physicochemical Properties and Activity, and In Vivo Biodistribution and Toxicity Studies
- PMID: 35336008
- PMCID: PMC8948811
- DOI: 10.3390/pharmaceutics14030633
Liposomal siRNA Formulations for the Treatment of Herpes Simplex Virus-1: In Vitro Characterization of Physicochemical Properties and Activity, and In Vivo Biodistribution and Toxicity Studies
Abstract
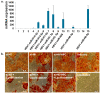
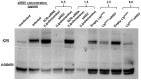
Herpes simplex virus-1 (HSV-1) is highly contagious, and there is a need for a therapeutic means to eradicate it. We have identified an siRNA (siHSV) that knocks down gene expression of the infected cell protein 0 (ICP0), which is important in the regulation of HSV infection. The selected siHSV was encapsulated in liposomes to overcome its poor stability, increase cell permeability, and prolonging siRNA circulation time. Several siRNAs against ICP0 have been designed and identified. We examined the role of various parameters, including formulation technique, lipids composition, and ratio. An optimal liposomal siHSV formulation (LipDOPE-siHSV) was characterized with desirable physiochemical properties, in terms of nano-size, low polydispersity index (PDI), neutral surface charge, high siHSV loading, spherical shape, high stability in physiologic conditions in vitro, and long-term shelf-life stability (>1 year, 4 °C). The liposomes exhibited profound internalization by human keratinocytes, no cytotoxicity in cell cultures, no detrimental effect on mice liver enzymes, and a gradual endo-lysosomal escape. Mice biodistribution studies in intact mice revealed accumulation, mainly in visceral organs but also in the trigeminal ganglion. The therapeutic potential of siHSV liposomes was demonstrated by significant antiviral activity both in the plaque reduction assay and in the 3D epidermis model, and the mechanism of action was validated by the reduction of ICP0 expression levels.
Keywords: 3D epidermis model; HSV-1; gene delivery; liposomes; nanomedicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources

