Tailoring Rational Manufacturing of Extemporaneous Compounding Oral Dosage Formulations with a Low Dose of Minoxidil
- PMID: 35336032
- PMCID: PMC8950007
- DOI: 10.3390/pharmaceutics14030658
Tailoring Rational Manufacturing of Extemporaneous Compounding Oral Dosage Formulations with a Low Dose of Minoxidil
Abstract
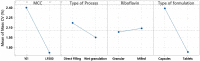
Low amounts of minoxidil in oral dosage forms are commonly prescribed as anti-alopecic pharmacological treatments. Side effects are usually related to individual susceptibility. However, poor drug content and mass uniformity can lead to a potential risk of overdosing, and higher chances to experience side effects. The impacts of four formulation variables on drug content and mass pharmaceutical quality attributes were studied with an experimental design at two levels. The first variable (A) was the particle size of the direct compression microcrystalline cellulose (MCC) used as a diluent (Avicel® PH 101 vs. LP 200). The second variable (B) was the type of production process (direct filling vs. wet granulation). The third variable (C) was the particle size of riboflavin added as a color mixture indicator agent (granular vs. milled). The fourth variable (D) was the type of oral solid dosage form (capsule vs. tablet). In half of the formulations, the mean minoxidil content and minoxidil uniformity were out of the specification limits of the Pharmacopoeia, demonstrating the importance of carefully selecting the excipients as well as the utilized process when manufacturing low oral dosage minoxidil formulations. The best minoxidil content uniformity was achieved when using MCC LP 200, wet granulation, granular riboflavin, and capsules. However, tablets are the recommended dosage form when utilizing Avicel® PH 101 or direct filling. Meeting these criteria, the content and mass uniformity are more likely to meet the specification limits of the Pharmacopeia. Techniques such as NIR spectroscopy should be implemented to control the quality of extemporaneous compounding formulations with a low dose of active ingredient.
Keywords: capsule; compounding; content; minoxidil; particle size; tablet; uniformity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jimenez-Cauhe J., Saceda-Corralo D., Rodrigues-Barata R., Moreno-Arrones O.M., Ortega-Quijano D., Fernandez-Nieto D., Jaen-Olasolo P., Vaño-Galvan S. Safety of low-dose oral minoxidil treatment for hair loss. A systematic review and pooled-analysis of individual patient data. Dermatol. Ther. 2020;33:e14106. doi: 10.1111/dth.14106. - DOI - PubMed
-
- Vañó-Galván S., Pirmez R., Hermosa-Gelbard A., Moreno-Arrones Ó.M., Saceda-Corralo D., Rodrigues-Barata R., Jimenez-Cauhe J., Koh W.L., Poa J.E., Jerjen R., et al. Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J. Am. Acad. Dermatol. 2021;84:1644–1651. doi: 10.1016/j.jaad.2021.02.054. - DOI - PubMed
-
- EMA . Design of Experiments. ICH Guideline Q8 (R2) EMA; London, UK: 2004. Document EMA/CHMP/ICH/167068/2004.
LinkOut - more resources
Full Text Sources
Miscellaneous

