Early Antibiotic Exposure Alters Intestinal Development and Increases Susceptibility to Necrotizing Enterocolitis: A Mechanistic Study
- PMID: 35336095
- PMCID: PMC8951210
- DOI: 10.3390/microorganisms10030519
Early Antibiotic Exposure Alters Intestinal Development and Increases Susceptibility to Necrotizing Enterocolitis: A Mechanistic Study
Abstract
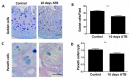
Increasing evidence suggests that prolonged antibiotic therapy in preterm infants is associated with increased mortality and morbidities, such as necrotizing enterocolitis (NEC), a devastating gastrointestinal pathology characterized by intestinal inflammation and necrosis. While a clinical correlation exists between antibiotic use and the development of NEC, the potential causality of antibiotics in NEC development has not yet been demonstrated. Here, we tested the effects of systemic standard-of-care antibiotic therapy for ten days on intestinal development in neonatal mice. Systemic antibiotic treatment impaired the intestinal development by reducing intestinal cell proliferation, villi height, crypt depth, and goblet and Paneth cell numbers. Oral bacterial challenge in pups who received antibiotics resulted in NEC-like intestinal injury in more than half the pups, likely due to a reduction in mucous-producing cells affecting microbial-epithelial interactions. These data support a novel mechanism that could explain why preterm infants exposed to prolonged antibiotics after birth have a higher incidence of NEC and other gastrointestinal disorders.
Keywords: antibiotics; intestinal development; intestinal permeability; microbiome; necrotizing enterocolitis; preterm infant.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures









References
-
- He W., Wang M.L., Jiang H.Q., Steppan C.M., Shin M.E., Thurnheer M.C., Cebra J.J., Lazar M.A., Wu G.D. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology. 2003;125:1388–1397. doi: 10.1016/j.gastro.2003.07.009. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

