Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task
- PMID: 35336793
- PMCID: PMC8945018
- DOI: 10.3390/biology11030419
Draining the Pleural Space: Lymphatic Vessels Facing the Most Challenging Task
Abstract
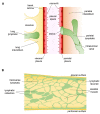
Lymphatic vessels exploit the mechanical stresses of their surroundings together with intrinsic rhythmic contractions to drain lymph from interstitial spaces and serosal cavities to eventually empty into the blood venous stream. This task is more difficult when the liquid to be drained has a very subatmospheric pressure, as it occurs in the pleural cavity. This peculiar space must maintain a very low fluid volume at negative hydraulic pressure in order to guarantee a proper mechanical coupling between the chest wall and lungs. To better understand the potential for liquid drainage, the key parameter to be considered is the difference in hydraulic pressure between the pleural space and the lymphatic lumen. In this review we collected old and new findings from in vivo direct measurements of hydraulic pressures in anaesthetized animals with the aim to better frame the complex physiology of diaphragmatic and intercostal lymphatics which drain liquid from the pleural cavity.
Keywords: breathing; chest wall; diaphragm; lung; lymphatic vessel; pleural cavity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Medical

