Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants
- PMID: 35336833
- PMCID: PMC8945163
- DOI: 10.3390/biology11030460
Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants
Abstract
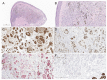
Deep penetrating nevus (DPN) is an uncommon acquired melanocytic lesion with a distinct histopathological appearance that typically behaves in an indolent manner. The lesion is characterized by a symmetrical proliferation of epithelioid to spindled melanocytes associated with abundant melanophages and wedge-shaped extension to the deep reticular dermis and subcutis. Pronounced cytologic atypia and mitotic figures are usually absent, which helps distinguish DPN from melanoma with a deep penetrating growth pattern. Recently, the concept of atypical DPN has been proposed for lesions that demonstrate borderline histomorphologic features and may be associated with lymph node deposits but lack the copy number aberrations typical of melanoma by either fluorescence in situ hybridization or comparative genomic hybridization. While most of these lesions have a favorable clinical course, rare lesions may progress to melanoma. In this review, we summarize the current literature on atypical DPNs with uncertain behavior/metastatic potential and outline the characteristics that distinguish these lesions from conventional DPN and melanoma with DPN-like features.
Keywords: deep penetrating nevus; melanocytic lesion; melanoma; metastatic; molecular; nevi.
Conflict of interest statement
The authors state that they have no conflict of interest.
Figures







References
-
- Elder D., Massi D., Scolier R., Willemze R.W. WHO Classification of Skin Tumors. WHO; Geneva, Switzerland: 2018.
-
- Scolyer R.A., Murali R., McCarthy S.W., Thompson J.F. Histologically ambiguous (“Borderline”) primary cutaneous melanocytic tumors: Approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch. Pathol. Lab. Med. 2010;134:1770–1777. doi: 10.5858/2009-0612-RAR.1. - DOI - PubMed
-
- North J.P., Garrido M.C., Kolaitis N.A., Leboit P.E., Mccalmont T.H., Bastian B.C. Fluorescence in situ hybridization as an ancillary tool in the diagnosis of ambiguous melanocytic neoplasms: A review of 804 cases. Am. J. Surg. Pathol. 2014;38:824–831. doi: 10.1097/PAS.0000000000000189. - DOI - PubMed
Publication types
Grants and funding
- Award ID 578728; https://doi.org/10.48050/pc.gr.80580/MRA/Melanoma Research Alliance/United States
- IRG/Institutional Research Grant and Start-up Funding from The University of Texas MD Anderson Cancer Center
- award number P30CA016672, which supports the MD Anderson Cancer Center Clinical Trials Office/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources

