Increased Heparanase Levels in Urine during Acute Puumala Orthohantavirus Infection Are Associated with Disease Severity
- PMID: 35336857
- PMCID: PMC8954369
- DOI: 10.3390/v14030450
Increased Heparanase Levels in Urine during Acute Puumala Orthohantavirus Infection Are Associated with Disease Severity
Abstract
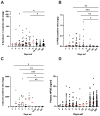
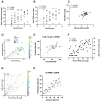
Old-world orthohantaviruses cause hemorrhagic fever with renal syndrome (HFRS), characterized by acute kidney injury (AKI) with transient proteinuria. It seems plausible that proteinuria during acute HFRS is mediated by the disruption of the glomerular filtration barrier (GFB) due to vascular leakage, a hallmark of orthohantavirus-caused diseases. However, direct infection of endothelial cells by orthohantaviruses does not result in increased endothelial permeability, and alternative explanations for vascular leakage and diminished GFB function are necessary. Vascular integrity is partly dependent on an intact endothelial glycocalyx, which is susceptible to cleavage by heparanase (HPSE). To understand the role of glycocalyx degradation in HFRS-associated proteinuria, we investigated the levels of HPSE in urine and plasma during acute, convalescent and recovery stages of HFRS caused by Puumala orthohantavirus. HPSE levels in urine during acute HFRS were significantly increased and strongly associated with the severity of AKI and other markers of disease severity. Furthermore, increased expression of HPSE was detected in vitro in orthohantavirus-infected podocytes, which line the outer surfaces of glomerular capillaries. Taken together, these findings suggest the local activation of HPSE in the kidneys of orthohantavirus-infected patients with the potential to disrupt the endothelial glycocalyx, leading to increased protein leakage through the GFB, resulting in high amounts of proteinuria.
Keywords: Puumala hantavirus; acute kidney injury; glycocalyx; heparanase; podocytes; proteinuria; syndecan–1.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

