Comparative Evaluation of Rapid Isothermal Amplification and Antigen Assays for Screening Testing of SARS-CoV-2
- PMID: 35336875
- PMCID: PMC8951466
- DOI: 10.3390/v14030468
Comparative Evaluation of Rapid Isothermal Amplification and Antigen Assays for Screening Testing of SARS-CoV-2
Abstract
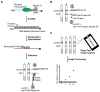
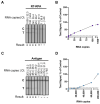
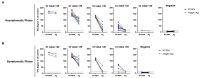
Human transmission of SARS-CoV-2 and emergent variants of concern continue to occur globally, despite mass vaccination campaigns. Public health strategies to reduce virus spread should therefore rely, in part, on frequent screening with rapid, inexpensive, and sensitive tests. We evaluated two digitally integrated rapid tests and assessed their performance using stored nasal swab specimens collected from individuals with or without COVID-19. An isothermal amplification assay combined with a lateral flow test had a limit of detection of 10 RNA copies per reaction, and a positive percent agreement (PPA)/negative percent agreement (NPA) during the asymptomatic and symptomatic phases of 100%/100% and 95.83/100%, respectively. Comparatively, an antigen-based lateral flow test had a limit of detection of 30,000 copies and a PPA/NPA during the asymptomatic and symptomatic phases of 82.86%/98.68% and 91.67/100%, respectively. Both the isothermal amplification and antigen-based lateral flow tests had optimized detection of SARS-CoV-2 during the peak period of transmission; however, the antigen-based test had reduced sensitivity in clinical samples with qPCR Ct values greater than 29.8. Low-cost, high-throughput screening enabled by isothermal amplification or antigen-based techniques have value for outbreak control.
Keywords: SARS-CoV-2; antigen test; isothermal molecular test; screening; surveillance; testing.
Conflict of interest statement
This work was supported by E25Bio, Inc. N.S., B.F.S. and B.B.H. are employed by E25Bio, Inc., a company that develops rapid diagnostic tests for infectious diseases. At the time of the study, X.Q. was employed by E25Bio, Inc. B.B.H. is an inventor on a U.S. Provisional Patent Application (63/189,502).
Figures
References
-
- El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., Campbell T.B., Clark J., Jackson L.A., Fichtenbaum C.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. - DOI - PMC - PubMed
-
- CDC Mitigation Measures for COVID-19 in Households and Markets in Non-US Low-Resource Settings. [(accessed on 18 December 2021)];2021 Available online: https://www.cdc.gov/coronavirus/2019-ncov/global-covid-19/global-urban-a....
-
- CDC Testing Strategies for SARS-CoV-2. [(accessed on 18 December 2021)];2021 Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/sars-cov2-testin....
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

