Enhanced SARS-CoV-2-Specific CD4+ T Cell Activation and Multifunctionality in Late Convalescent COVID-19 Individuals
- PMID: 35336918
- PMCID: PMC8954911
- DOI: 10.3390/v14030511
Enhanced SARS-CoV-2-Specific CD4+ T Cell Activation and Multifunctionality in Late Convalescent COVID-19 Individuals
Abstract
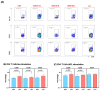
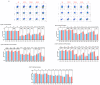
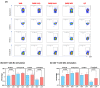
Background: Examination of CD4+ T cell responses during the natural course of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection offers useful information for the improvement of vaccination strategies against this virus and the protective effect of these T cells. Methods: We characterized the SARS-CoV-2-specific CD4+ T cell activation marker, multifunctional cytokine and cytotoxic marker expression in recovered coronavirus disease 2019 (COVID-19) individuals. Results: CD4+ T-cell responses in late convalescent (>6 months of diagnosis) individuals are characterized by elevated frequencies of activated as well as mono, dual- and multi-functional Th1 and Th17 CD4+ T cells in comparison to early convalescent (<1 month of diagnosis) individuals following stimulation with SARS-CoV-2-specific antigens. Similarly, the frequencies of cytotoxic marker expressing CD4+ T cells were also enhanced in late convalescent compared to early convalescent individuals. Conclusion: Our findings from a low-to middle-income country suggest protective adaptive immune responses following natural infection of SARS-CoV-2 are elevated even at six months following initial symptoms, indicating the CD4+ T cell mediated immune protection lasts for six months or more in natural infection.
Keywords: CD4+ T cells; COVID-19; SARS-CoV-2; cytokines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sekine T., Perez-Potti A., Rivera-Ballesteros O., Stralin K., Gorin J.B., Olsson A., Llewellyn-Lacey S., Kamal H., Bogdanovic G., Muschiol S., et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell. 2020;183:158–168.e14. doi: 10.1016/j.cell.2020.08.017. - DOI - PMC - PubMed
-
- Guan W.J., Zhong N.S. Clinical Characteristics of Covid-19 in China. Reply. N. Engl. J. Med. 2020;382:1861–1862. - PubMed
-
- Sherina N., Piralla A., Du L., Wan H., Kumagai-Braesch M., Andrell J., Braesch-Andersen S., Cassaniti I., Percivalle E., Sarasini A., et al. Persistence of SARS-CoV-2-specific B and T cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med. 2021;2:281–295.e4. doi: 10.1016/j.medj.2021.02.001. - DOI - PMC - PubMed
-
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. - DOI - PMC - PubMed
-
- Mathew D., Giles J.R., Baxter A.E., Oldridge D.A., Greenplate A.R., Wu J.E., Alanio C., Kuri-Cervantes L., Pampena M.B., D’Andrea K., et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369:eabc8511. doi: 10.1126/science.abc8511. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

