OraSure InteliSwab™ Rapid Antigen Test Performance with the SARS-CoV-2 Variants of Concern-Alpha, Beta, Gamma, Delta, and Omicron
- PMID: 35336950
- PMCID: PMC8951130
- DOI: 10.3390/v14030543
OraSure InteliSwab™ Rapid Antigen Test Performance with the SARS-CoV-2 Variants of Concern-Alpha, Beta, Gamma, Delta, and Omicron
Abstract
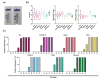
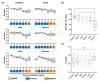
The emergence of SARS-CoV-2 in the human population and the resulting COVID-19 pandemic have led to the development of various diagnostic tests. The OraSure InteliSwab™ COVID-19 Rapid Test is a recently developed and FDA emergency use-authorized rapid antigen-detecting test that functions as a lateral flow device targeting the nucleocapsid protein. Due to SARS-CoV-2 evolution, there is a need to evaluate the sensitivity of rapid antigen-detecting tests for new variants, especially variants of concern such as Omicron. In this study, the sensitivity of the OraSure InteliSwab™ Test was investigated using cultured strains of the known variants of concern (VOCs, Alpha, Beta, Gamma, Delta, and Omicron) and the ancestral lineage (lineage A). Based on dilution series in cell culture medium, an approximate limit of detection for each variant was determined. The OraSure InteliSwab™ Test showed an overall comparable performance using recombinant nucleocapsid protein and different cultured variants, with recorded limits of detection ranging between 3.77 × 105 and 9.13 × 105 RNA copies/mL. Finally, the sensitivity was evaluated using oropharyngeal swabs from Syrian golden hamsters inoculated with the six VOCs. Ultimately, the OraSure InteliSwab™ COVID-19 Rapid Test showed no decrease in sensitivity between the ancestral SARS-CoV-2 strain and any VOCs including Omicron.
Keywords: COVID-19; Delta; Omicron; SARS-CoV-2; rapid antigen-detecting test; variants of concern.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Update of
-
OraSure InteliSwab ® Rapid Antigen Test performance with the SARS-CoV-2 Variants of Concern Alpha, Beta, Gamma, Delta, and Omicron.medRxiv [Preprint]. 2022 Feb 4:2022.02.02.22270254. doi: 10.1101/2022.02.02.22270254. medRxiv. 2022. Update in: Viruses. 2022 Mar 06;14(3):543. doi: 10.3390/v14030543. PMID: 35169818 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

