West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017-2020)
- PMID: 35336976
- PMCID: PMC8951896
- DOI: 10.3390/v14030569
West Nile Virus Lineage 2 Spreads Westwards in Europe and Overwinters in North-Eastern Spain (2017-2020)
Abstract
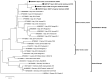
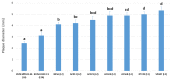
West Nile virus lineage 2 (WNV-L2) emerged in Europe in 2004; since then, it has spread across the continent, causing outbreaks in humans and animals. During 2017 and 2020, WNV-L2 was detected and isolated from four northern goshawks in two provinces of Catalonia (north-eastern Spain). In order to characterise the first Spanish WNV-L2 isolates and elucidate the potential overwintering of the virus in this Mediterranean region, complete genome sequencing, phylogenetic analyses, and a study of phenotypic characterisation were performed. Our results showed that these Spanish isolates belonged to the central-southern WNV-L2 clade. In more detail, they were related to the Lombardy cluster that emerged in Italy in 2013 and has been able to spread westwards, causing outbreaks in France (2018) and Spain (2017 and 2020). Phenotypic characterisation performed in vitro showed that these isolates presented characteristics corresponding to strains of moderate to high virulence. All these findings evidence that these WNV-L2 strains have been able to circulate and overwinter in the region, and are pathogenic, at least in northern goshawks, which seem to be very susceptible to WNV infection and may be good indicators of WNV-L2 circulation. Due to the increasing number of human and animal cases in Europe in the last years, this zoonotic flavivirus should be kept under extensive surveillance, following a One-Health approach.
Keywords: Europe; Spain; West Nile virus; avian host; lineage-2; northern goshawk; overwintering; phylogeny.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- García San Miguel Rodríguez-Alarcón L., Fernández-Martínez B., Sierra Moros M.J., Vázquez A., Julián Pachés P., García Villacieros E., Gómez Martín M.B., Figuerola Borras J., Lorusso N., Ramos Aceitero J.M., et al. Unprecedented Increase of West Nile Virus Neuroinvasive Disease, Spain, Summer 2020. Eurosurveillance. 2021;26:2002010. doi: 10.2807/1560-7917.ES.2021.26.19.2002010. - DOI - PMC - PubMed
-
- Rizzoli A., Jimenez-Clavero M.A., Barzon L., Cordioli P., Figuerola J., Koraka P., Martina B., Moreno A., Nowotny N., Pardigon N., et al. The Challenge of West Nile Virus in Europe : Knowledge Gaps and Research Priorities. Eurosurveillance. 2015;20:21135. doi: 10.2807/1560-7917.ES2015.20.20.21135. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

