Viral Shrimp Diseases Listed by the OIE: A Review
- PMID: 35336992
- PMCID: PMC8953307
- DOI: 10.3390/v14030585
Viral Shrimp Diseases Listed by the OIE: A Review
Abstract
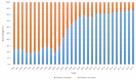
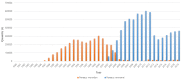
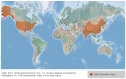
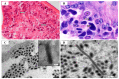
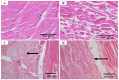
Shrimp is one of the most valuable aquaculture species globally, and the most internationally traded seafood product. Consequently, shrimp aquaculture practices have received increasing attention due to their high value and levels of demand, and this has contributed to economic growth in many developing countries. The global production of shrimp reached approximately 6.5 million t in 2019 and the shrimp aquaculture industry has consequently become a large-scale operation. However, the expansion of shrimp aquaculture has also been accompanied by various disease outbreaks, leading to large losses in shrimp production. Among the diseases, there are various viral diseases which can cause serious damage when compared to bacterial and fungi-based illness. In addition, new viral diseases occur rapidly, and existing diseases can evolve into new types. To address this, the review presented here will provide information on the DNA and RNA of shrimp viral diseases that have been designated by the World Organization for Animal Health and identify the latest shrimp disease trends.
Keywords: DNA and RNA virus; OIE; shrimp disease; viral disease.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






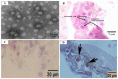
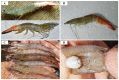



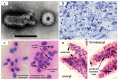



References
-
- Roy S., Bossier P., Norouzitallab P., Vanrompay D. Trained immunity and perspectives for shrimp aquaculture. Rev. Aquac. 2020;12:2351–2370. doi: 10.1111/raq.12438. - DOI
-
- Manan H., Ikhwanuddin M. Triploid induction in penaeid shrimps aquaculture: A review. Rev. Aquac. 2021;13:619–631. doi: 10.1111/raq.12489. - DOI
-
- Morshed M., Islam M.S., Lohano H.D., Shyamsundar P. Production externalities of shrimp aquaculture on paddy farming in coastal Bangladesh. Agric. Water. Manag. 2020;238:106213. doi: 10.1016/j.agwat.2020.106213. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

