Relationship between HIV-1 Gag Multimerization and Membrane Binding
- PMID: 35337029
- PMCID: PMC8949992
- DOI: 10.3390/v14030622
Relationship between HIV-1 Gag Multimerization and Membrane Binding
Abstract
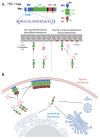
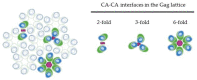
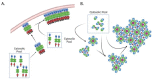
HIV-1 viral particle assembly occurs specifically at the plasma membrane and is driven primarily by the viral polyprotein Gag. Selective association of Gag with the plasma membrane is a key step in the viral assembly pathway, which is traditionally attributed to the MA domain. MA regulates specific plasma membrane binding through two primary mechanisms including: (1) specific interaction of the MA highly basic region (HBR) with the plasma membrane phospholipid phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2], and (2) tRNA binding to the MA HBR, which prevents Gag association with non-PI(4,5)P2 containing membranes. Gag multimerization, driven by both CA-CA inter-protein interactions and NC-RNA binding, also plays an essential role in viral particle assembly, mediating the establishment and growth of the immature Gag lattice on the plasma membrane. In addition to these functions, the multimerization of HIV-1 Gag has also been demonstrated to enhance its membrane binding activity through the MA domain. This review provides an overview of the mechanisms regulating Gag membrane binding through the MA domain and multimerization through the CA and NC domains, and examines how these two functions are intertwined, allowing for multimerization mediated enhancement of Gag membrane binding.
Keywords: Gag; HIV-1; capsid; genomic RNA binding; matrix; membrane binding; multimerization; nucleocapsid; particle assembly; tRNA binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Relationships between MA-RNA Binding in Cells and Suppression of HIV-1 Gag Mislocalization to Intracellular Membranes.J Virol. 2019 Nov 13;93(23):e00756-19. doi: 10.1128/JVI.00756-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511376 Free PMC article.
-
Molecular Determinants Directing HIV-1 Gag Assembly to Virus-Containing Compartments in Primary Macrophages.J Virol. 2016 Sep 12;90(19):8509-19. doi: 10.1128/JVI.01004-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440886 Free PMC article.
-
Membrane Binding of HIV-1 Matrix Protein: Dependence on Bilayer Composition and Protein Lipidation.J Virol. 2016 Apr 14;90(9):4544-4555. doi: 10.1128/JVI.02820-15. Print 2016 May. J Virol. 2016. PMID: 26912608 Free PMC article.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
-
Role of the nucleocapsid region in HIV-1 Gag assembly as investigated by quantitative fluorescence-based microscopy.Virus Res. 2014 Nov 26;193:78-88. doi: 10.1016/j.virusres.2014.06.009. Epub 2014 Jul 9. Virus Res. 2014. PMID: 25016037 Review.
Cited by
-
The HBV large envelope protein initiates virion assembly by recruiting capsids at membrane rich domains related to late endosome.Cell Mol Life Sci. 2025 Mar 24;82(1):128. doi: 10.1007/s00018-025-05574-3. Cell Mol Life Sci. 2025. PMID: 40128454 Free PMC article.
-
HIV-1 Gag Binds the Multi-Aminoacyl-tRNA Synthetase Complex via the EPRS Subunit.Viruses. 2023 Feb 8;15(2):474. doi: 10.3390/v15020474. Viruses. 2023. PMID: 36851687 Free PMC article.
-
HIV-1 Gag targeting to the plasma membrane reorganizes sphingomyelin-rich and cholesterol-rich lipid domains.Nat Commun. 2023 Nov 21;14(1):7353. doi: 10.1038/s41467-023-42994-w. Nat Commun. 2023. PMID: 37990014 Free PMC article.
-
Analysis of Arc/Arg3.1 Oligomerization In Vitro and in Living Cells.Int J Mol Sci. 2024 Jun 12;25(12):6454. doi: 10.3390/ijms25126454. Int J Mol Sci. 2024. PMID: 38928159 Free PMC article.
-
Influence of HIV-1 Genomic RNA on the Formation of Gag Biomolecular Condensates.J Mol Biol. 2023 Aug 15;435(16):168190. doi: 10.1016/j.jmb.2023.168190. Epub 2023 Jun 27. J Mol Biol. 2023. PMID: 37385580 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

