Enteric Viruses Nucleic Acids Distribution along the Digestive Tract of Rhesus Macaques with Idiopathic Chronic Diarrhea
- PMID: 35337045
- PMCID: PMC8951234
- DOI: 10.3390/v14030638
Enteric Viruses Nucleic Acids Distribution along the Digestive Tract of Rhesus Macaques with Idiopathic Chronic Diarrhea
Abstract
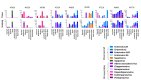
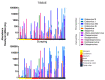
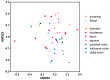
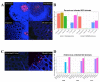
Idiopathic chronic diarrhea (ICD) is a little understood common clinical problem in captive rhesus macaques claiming 33% of medical culls unrelated to research. The eukaryotic virome in digestive tract tissues collected at necropsy from nine animals with ICD was characterized using viral metagenomics. We compared the distribution of viral reads in tissues and mucosal scrapings from the stomach, duodenum, jejunum, ileum, and the proximal, transverse, and distal colons. In situ hybridization (ISH) using viral probes were performed on fixed tissues. Deep sequencing revealed multiple viruses in the Parvoviridae and Picornaviridae family. Tissues and mucosal scraping from the same locations showed closely related viral reads contents while different gut tissues from the same animal varied widely. ISH showed punctuated staining for both RNA and DNA viruses in the distal colon. Parvovirus staining was also detected in the stomach/duodenum/jejunum in distinct oval-shaped structures. The location of enteric viral nucleic acid differed widely between different viral families and along the length of the digestive tract.
Keywords: biogeography; idiopathic chronic diarrhea; viral metagenomics; virome.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Sestak K., Merritt C.K., Borda J., Saylor E., Schwamberger S.R., Cogswell F., Didier E.S., Didier P.J., Plauche G., Bohm R.P., et al. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect. Immun. 2003;71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003. - DOI - PMC - PubMed

