Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route
- PMID: 35337079
- PMCID: PMC8955068
- DOI: 10.3390/ph15030281
Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route
Abstract
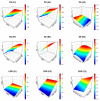
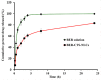
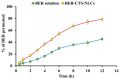
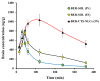
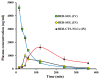
This research aimed to design, optimize, and evaluate berberine-laden nanostructured lipid carriers overlaid with chitosan (BER-CTS-NLCs) for efficient brain delivery via the intranasal route. The nanostructured lipid carriers containing berberine (BER-NLCs) were formulated via hot homogenization and ultrasonication strategy and optimized for the influence of a variety of causal variables, including the amount of glycerol monostearate (solid lipid), poloxamer 407 (surfactant) concentration, and oleic acid (liquid lipid) amount, on size of the particles, entrapment, and the total drug release after 24 h. The optimal BER-NLCs formulation was then coated with chitosan. Their diameter, in vitro release, surface charge, morphology, ex vivo permeability, pH, histological, and in vivo (pharmacokinetics and brain uptake) parameters were estimated. BER-CTS-NLCs had a size of 180.9 ± 4.3 nm, sustained-release properties, positive surface charge of 36.8 mV, and augmented ex-vivo permeation via nasal mucosa. The histopathological assessment revealed that the BER-CTS-NLCs system is safe for nasal delivery. Pharmacokinetic and brain accumulation experiments showed that animals treated intranasally with BER-CTS-NLCs had substantially greater drug levels in the brain. The ratios of BER brain/blood levels at 30 min, AUCbrain/AUCblood, drug transport percentage, and drug targeting efficiency for BER-CTS-NLCs (IN) were higher compared to BER solution (IN), suggesting enhanced brain targeting. The optimized nanoparticulate system is speculated to be a successful approach for boosting the effect of BER in treating CNS diseases, such as Alzheimer's disease, through intranasal therapy.
Keywords: biodistribution; brain targeting; coatings; intranasal; lipidic nanoparticles; nutraceuticals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lee D.-H., Park K.-I., Park H.-S., Kang S.-R., Nagappan A., Kim J.-A., Kim E.-H., Lee W.-S., Hah Y.-S., Chung H.-J. Flavonoids isolated from Korea Citrus aurantium L. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. Evid.-Based Complement. Altern. Med. 2012;2012:515901. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

