Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations
- PMID: 35337124
- PMCID: PMC8955762
- DOI: 10.3390/ph15030326
Press-Coated Aceclofenac Tablets for Pulsatile Drug Delivery: Formulation and In Vitro Evaluations
Abstract
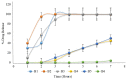
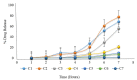
The symptoms of some diseases show circadian rhythms, such as the morning stiffness associated with pain at the time of awakening in rheumatoid arthritis. Therapy for such diseases doesn't require immediate release or sustained release of medicament. In such therapies, pulsatile drug release is more suitable with a programmed drug release. The purpose of this research was to formulate press-coated aceclofenac tablets for pulsatile drug delivery with a distinct delay time of no drug release and release of the drug when it is more likely desired (i.e., after 5 to 6 h). Immediate release core tablets having aceclofenac were formulated. Three formulations, F1, F2, and F3, were prepared with variable concentrations of sodium croscarmellose. Pre- and post-compression tests were performed on the core tablets. The selection criteria included the lowest disintegration time as a requirement of pulsatile drug delivery with an immediate release core and a delayed release coat. The disintegration times of F1, F2, and F3 were 120 s, 60 s, and 15 s, respectively. Therefore, the F3 formulation was selected as the core tablet formulation because it had the shortest disintegration time (15 s). The core tablets were press-coated using different polymers, such as HPMC K100M, Eudragit L100, HEC, and HPMC E5. The polymers were used in the coatings to hinder the release of the core for the desired time. 36 formulations of polymer were prepared: A1 to A10 had HPMC K100M and Avicel PH102; formulations B1 to B6 had HPMC K100M, Eudragit L100, and Avicel PH102; formulations C1 to C7 had HPMC K100M and hydroxyethyl cellulose; formulations D1 to D7 had HPMC K100M and HPMC E5; and formulations E1 to E6 had changed the coating weight of the formulation used for D6 (having HPMC K100M and HPMC E5 in the ratio of 12.5% to 87.5%). Evaluations of the press-coated tablets were carried out through thickness, hardness, weight variation, friability, and in vitro dissolution tests. These parameters concluded that the formulation of E6, having HPMC K100M and HPMC E5 in the ratio of 12.5% to 87.5% at 600 mg weight, was the most optimum formulation as it showed 3.5% drug release after 4 h, 21.4% drug release after 5 h, and 99.27% drug release after 6 h.
Keywords: FTIR; HEC; HPMC; NSAIDs; pulsatile drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Investigating effects of hydroxypropyl methylcellulose (HPMC) molecular weight grades on lag time of press-coated ethylcellulose tablets.Pharm Dev Technol. 2016 Nov;21(7):794-802. doi: 10.3109/10837450.2015.1055767. Epub 2015 Jun 23. Pharm Dev Technol. 2016. PMID: 26100758
-
Development of press-coated, floating-pulsatile drug delivery of lisinopril.Sci Pharm. 2014 Apr 14;82(2):423-40. doi: 10.3797/scipharm.1301-27. Print 2014 Apr-Jun. Sci Pharm. 2014. PMID: 24959410 Free PMC article.
-
Release characteristics and in vitro-in vivo correlation of pulsatile pattern for a pulsatile drug delivery system activated by membrane rupture via osmotic pressure and swelling.Eur J Pharm Biopharm. 2008 Sep;70(1):289-301. doi: 10.1016/j.ejpb.2008.03.021. Epub 2008 Apr 22. Eur J Pharm Biopharm. 2008. PMID: 18539015 Clinical Trial.
-
Quality by design-based development and in vitro evaluation of dual release tablet of etoricoxib and thiocolchicoside: A novel chronotherapeutic approach for arthritis pain management.Ann Pharm Fr. 2024 Nov;82(6):1103-1117. doi: 10.1016/j.pharma.2024.07.004. Epub 2024 Jul 11. Ann Pharm Fr. 2024. PMID: 39002854
-
Aceclofenac fast dispersible tablet formulations: Effect of different concentration levels of Avicel PH102 on the compactional, mechanical and drug release characteristics.PLoS One. 2020 Feb 12;15(2):e0223201. doi: 10.1371/journal.pone.0223201. eCollection 2020. PLoS One. 2020. PMID: 32050259 Free PMC article.
Cited by
-
The Development of Eletriptan Hydrobromide Immediate Release Buccal Films Using Central Composite Rotatable Design: An In Vivo and In Vitro Approach.Polymers (Basel). 2022 Sep 23;14(19):3981. doi: 10.3390/polym14193981. Polymers (Basel). 2022. PMID: 36235932 Free PMC article.
References
-
- Moon A., Kondawar M., Shah R. Formulation and evaluation of press-coated indomethacin tablets for pulsatile drug delivery system. J. Pharm. Res. 2011;4:564–566.
-
- Satani R., Chotaliya M., Raval M., Sheth N. Review on recent trends in press-coated pulse drug delivery system. Int. Bull. Drug Res. 2014;4:60–91.
-
- Prasanth V., Mitesh P., Sam T., Abin A. Formulation and evaluation of enteric coated time release press coated tablets of theophylline for chronopharmacotherapy. Pharm. Lett. 2012;4:599–606.
-
- Arslan S.A., Tirnaksiz F. A nonsteroidal antiinflammatory drug: Aceclofenac. FABA J. Pharm. Sci. 2010;35:105–118.
-
- Bushra R., Shoaib M.H., Naeem M.I., Aslam N. Aceclofenac: A new effective and safe NSAID. Int. J. Drug Deliv. Technol. 2013;42:1.
LinkOut - more resources
Full Text Sources
Miscellaneous

