Formulation of Chitosan-Coated Brigatinib Nanospanlastics: Optimization, Characterization, Stability Assessment and In-Vitro Cytotoxicity Activity against H-1975 Cell Lines
- PMID: 35337145
- PMCID: PMC8948618
- DOI: 10.3390/ph15030348
Formulation of Chitosan-Coated Brigatinib Nanospanlastics: Optimization, Characterization, Stability Assessment and In-Vitro Cytotoxicity Activity against H-1975 Cell Lines
Abstract
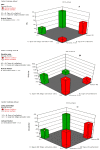
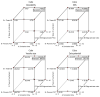
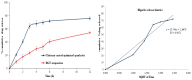
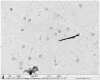
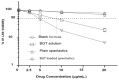
The purpose of the current study was to develop Brigatinib (BGT)-loaded nanospanlastics (BGT-loaded NSPs) (S1-S13) containing Span 60 with different edge activators (Tween 80 and Pluronic F127) and optimized based on the vesicle size, zeta potential (ZP), and percent entrapment efficiency (%EE) using Design-Expert® software. The optimum formula was recommended with desirability of 0.819 and composed of Span-60:Tween 80 at a ratio of 4:1 and 10 min as a sonication time (S13). It showed predicted EE% (81.58%), vesicle size (386.55 nm), and ZP (-29.51 mv). The optimized nanospanlastics (S13) was further coated with chitosan and further evaluated for Differential Scanning Calorimetry (DSC), X-ray Diffraction (XRD), in vitro release, Transmission Electron Microscopy (TEM), stability and in-vitro cytotoxicity studies against H-1975 lung cancer cell lines. The DSC and XRD revealed complete encapsulation of the drug. TEM imagery revealed spherical nanovesicles with a smooth surface. Also, the coated formula showed high stability for three months in two different conditions. Moreover, it resulted in improved and sustained drug release than free BGT suspension and exhibited Higuchi kinetic release mechanism. The cytotoxic activity of BGT-loaded SPs (S13) was enhanced three times in comparison to free the BGT drug against the H-1975 cell lines. Overall, these results confirmed that BGT-loaded SPs could be a promising nanocarrier to improve the anticancer efficacy of BGT.
Keywords: brigatinib; chitosan; cytotoxicity; nanospanlastics; optimization; sustained release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kim D.W., Tiseo M., Ahn M.J., Reckamp K.L., Hansen K.H., Kim S.W., Leighl N.B. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J. Clin. Oncol. 2017;35:2490–2498. doi: 10.1200/JCO.2016.71.5904. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

