In Vitro and In Vivo Antiviral Studies of New Heteroannulated 1,2,3-Triazole Glycosides Targeting the Neuraminidase of Influenza A Viruses
- PMID: 35337148
- PMCID: PMC8950700
- DOI: 10.3390/ph15030351
In Vitro and In Vivo Antiviral Studies of New Heteroannulated 1,2,3-Triazole Glycosides Targeting the Neuraminidase of Influenza A Viruses
Abstract
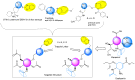
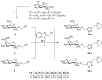
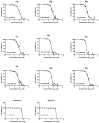
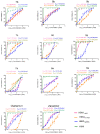
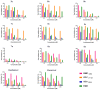
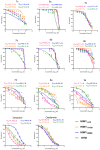
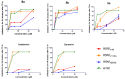
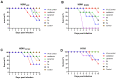
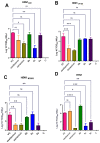
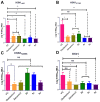
There is an urgent need to develop and synthesize new anti-influenza drugs with activity against different strains, resistance to mutations, and suitability for various populations. Herein, we tested in vitro and in vivo the antiviral activity of new 1,2,3-triazole glycosides incorporating benzimidazole, benzooxazole, or benzotriazole cores synthesized by using a click approach. The Cu-catalyzation strategy consisted of 1,3-dipolar cycloaddition of the azidoalkyl derivative of the respective heterocyclic and different glycosyl acetylenes with five or six carbon sugar moieties. The antiviral activity of the synthesized glycosides against wild-type and neuraminidase inhibitor resistant strains of the avian influenza H5N1 and human influenza H1N1 viruses was high in vitro and in mice. Structure-activity relationship studies showed that varying the glycosyl moiety in the synthesized glycosides enhanced antiviral activity. The compound (2R,3R,4S,5R)-2-((1-(Benzo[d]thiazol-2-ylmethyl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate (Compound 9c) had a 50% inhibitory concentration (IC50) = 2.280 µM and a ligand lipophilic efficiency (LLE) of 6.84. The compound (2R,3R,4S,5R)-2-((1-((1H-Benzo[d]imidazol-2-yl)methyl)-1H-1,2,3-triazol-4-yl)methoxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate had IC50 = 2.75 µM and LLE = 7.3 after docking analysis with the H5N1 virus neuraminidase. Compound 9c achieved full protection from H1N1 infection and 80% protection from H5N1 in addition to a high binding energy with neuraminidase and was safe in vitro and in vivo. This compound is suitable for further clinical studies as a new neuraminidase inhibitor.
Keywords: antiviral; avian H5N1; glycosides; heterocyclic; human H1N1; neuraminidase inhibitor.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
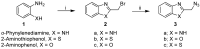

References
-
- Abed Y., Baz M., Boivin G. Impact of neuraminidase mutations conferring influenza resistance to neuraminidase inhibitors in the N1 and N2 genetic backgrounds. Antivir. Ther. 2006;11:971–976. - PubMed
-
- Prachanronarong K.L., Canale A.S., Liu P., Somasundaran M., Hou S., Poh Y.-P., Han T., Zhu Q., Renzette N., Zeldovich K.B., et al. Mutations in Influenza A Virus Neuraminidase and Hemagglutinin Confer Resistance against a Broadly Neutralizing Hemagglutinin Stem Antibody. J. Virol. 2019;93:e01639-18. doi: 10.1128/JVI.01639-18. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

