Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production
- PMID: 35337181
- PMCID: PMC8954882
- DOI: 10.3390/ph15030384
Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production
Erratum in
-
Correction: Alfei, S.; Zuccari, G. Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production. Pharmaceuticals 2022, 15, 384.Pharmaceuticals (Basel). 2022 Apr 25;15(5):526. doi: 10.3390/ph15050526. Pharmaceuticals (Basel). 2022. PMID: 35631473 Free PMC article.
Abstract
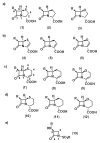
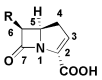
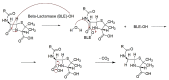
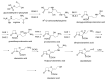
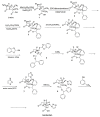
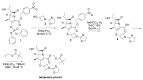
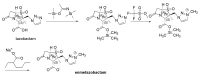
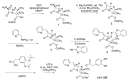
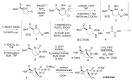
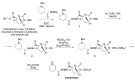
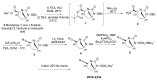
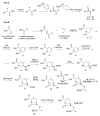
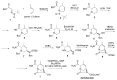
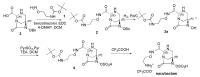
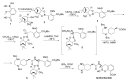
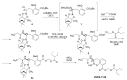
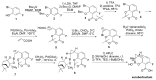
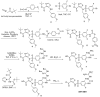
The increasing emergence of bacteria producing β-lactamases enzymes (BLEs), able to inactivate the available β-lactam antibiotics (BLAs), causing the hydrolytic opening of their β-lactam ring, is one of the global major warnings. According to Ambler classification, BLEs are grouped in serine-BLEs (SBLEs) of class A, C, and D, and metal-BLEs (MBLEs) of class B. A current strategy to restore no longer functioning BLAs consists of associating them to β-lactamase enzymes inhibitors (BLEsIs), which, interacting with BLEs, prevent them hydrolyzing to the associated antibiotic. Worryingly, the inhibitors that are clinically approved are very few and inhibit only most of class A and C SBLEs, leaving several class D and all MBLEs of class B untouched. Numerous non-clinically approved new molecules are in development, which have shown broad and ultra-broad spectrum of action, some of them also being active on the New Delhi metal-β-lactamase-1 (NDM-1), which can hydrolyze all available BLAs except for aztreonam. To not duplicate the existing review concerning this topic, we have herein examined BLEsIs by a chemistry approach. To this end, we have reviewed both the long-established synthesis adopted to prepare the old BLEsIs, those proposed to achieve the BLEsIs that are newly approved, and those recently reported to prepare the most relevant molecules yet in development, which have shown high potency, providing for each synthesis the related reaction scheme.
Keywords: metal-β-lactamases; multi-drug resistant (MDR) bacteria; optimized synthetic procedures; serine β-lactamases; β-lactam antibiotics; β-lactamase enzymes; β-lactamase enzymes inhibitors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
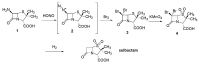





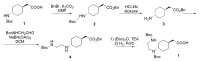
Similar articles
-
β-Lactam Antibiotics and β-Lactamase Enzymes Inhibitors, Part 2: Our Limited Resources.Pharmaceuticals (Basel). 2022 Apr 13;15(4):476. doi: 10.3390/ph15040476. Pharmaceuticals (Basel). 2022. PMID: 35455473 Free PMC article. Review.
-
Class D β-lactamases: a reappraisal after five decades.Acc Chem Res. 2013 Nov 19;46(11):2407-15. doi: 10.1021/ar300327a. Epub 2013 Jul 31. Acc Chem Res. 2013. PMID: 23902256 Free PMC article. Review.
-
Beta-lactamase inhibitors: the story so far.Curr Med Chem. 2009;16(28):3740-65. doi: 10.2174/092986709789104957. Curr Med Chem. 2009. PMID: 19747143 Review.
-
Recent advances in the development of β-lactamase inhibitors.J Microbiol. 2020 Aug;58(8):633-647. doi: 10.1007/s12275-020-0285-z. Epub 2020 Jul 27. J Microbiol. 2020. PMID: 32720096 Review.
-
[Mechanisms of resistance in Enterobacteriaceae towards beta-lactamase antibiotics].Acta Med Croatica. 2004;58(4):307-12. Acta Med Croatica. 2004. PMID: 15700687 Croatian.
Cited by
-
Challenges of Carbapenem-Resistant Enterobacteriaceae in the Development of New β-Lactamase Inhibitors and Antibiotics.Antibiotics (Basel). 2025 Jun 7;14(6):587. doi: 10.3390/antibiotics14060587. Antibiotics (Basel). 2025. PMID: 40558176 Free PMC article. Review.
-
Correction: Alfei, S.; Zuccari, G. Recommendations to Synthetize Old and New β-Lactamases Inhibitors: A Review to Encourage Further Production. Pharmaceuticals 2022, 15, 384.Pharmaceuticals (Basel). 2022 Apr 25;15(5):526. doi: 10.3390/ph15050526. Pharmaceuticals (Basel). 2022. PMID: 35631473 Free PMC article.
-
Antibiotic Adjuvants: A Versatile Approach to Combat Antibiotic Resistance.ACS Omega. 2023 Mar 14;8(12):10757-10783. doi: 10.1021/acsomega.3c00312. eCollection 2023 Mar 28. ACS Omega. 2023. PMID: 37008128 Free PMC article. Review.
-
Synthesized Bis-Triphenyl Phosphonium-Based Nano Vesicles Have Potent and Selective Antibacterial Effects on Several Clinically Relevant Superbugs.Nanomaterials (Basel). 2024 Aug 15;14(16):1351. doi: 10.3390/nano14161351. Nanomaterials (Basel). 2024. PMID: 39195389 Free PMC article.
-
Antimicrobial Peptides and Cationic Nanoparticles: A Broad-Spectrum Weapon to Fight Multi-Drug Resistance Not Only in Bacteria.Int J Mol Sci. 2022 May 29;23(11):6108. doi: 10.3390/ijms23116108. Int J Mol Sci. 2022. PMID: 35682787 Free PMC article. Review.
References
-
- List of β-lactam Antibiotics. From Wikipedia, the Free Encyclopedia. [(accessed on 8 February 2022)]. Available online: https://en.wikipedia.org/wiki/List_of_%CE%B2-lactam_antibiotics.
Publication types
LinkOut - more resources
Full Text Sources

