Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
- PMID: 35337266
- PMCID: PMC8953967
- DOI: 10.1186/s12879-022-07285-7
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics
Abstract
Background: There is an urgent need to expand testing for SARS-CoV-2 and other respiratory pathogens as the global community struggles to control the COVID-19 pandemic. Current diagnostic methods can be affected by supply chain bottlenecks and require the assistance of medical professionals, impeding the implementation of large-scale testing. Self-collection of saliva may solve these problems, as it can be completed without specialized training and uses generic materials.
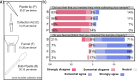
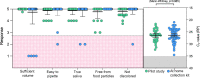
Methods: We observed 30 individuals who self-collected saliva using four different collection devices and analyzed their feedback. Two of these devices, a funnel and bulb pipette, were used to evaluate at-home saliva collection by 60 individuals. SARS-CoV-2-spiked saliva samples were subjected to temperature cycles designed to simulate the conditions the samples might be exposed to during the summer and winter seasons and sensitivity of detection was evaluated.
Results: All devices enabled the safe, unsupervised self-collection of saliva. The quantity and quality of the samples received were acceptable for SARS-CoV-2 diagnostic testing, as determined by human RNase P detection. There was no significant difference in SARS-CoV-2 nucleocapsid gene (N1) detection between the freshly spiked samples and those incubated with the summer and winter profiles.
Conclusion: We demonstrate inexpensive, generic, buffer free collection devices suitable for unsupervised and home saliva self-collection.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Update of
-
Evaluation of saliva self-collection devices for SARS-CoV-2 diagnostics.medRxiv [Preprint]. 2021 Jul 3:2021.02.01.21250946. doi: 10.1101/2021.02.01.21250946. medRxiv. 2021. Update in: BMC Infect Dis. 2022 Mar 25;22(1):284. doi: 10.1186/s12879-022-07285-7. PMID: 33564787 Free PMC article. Updated. Preprint.
References
-
- Hanson KE, Barker AP, Hillyard DR, Gilmore N, Barrett JW, Orlandi RR, et al. Self-collected anterior nasal and saliva specimens versus health care worker-collected nasopharyngeal swabs for the molecular detection of SARS-CoV-2. J Clin Microbiol. 2020;58(11):e0182420. doi: 10.1128/JCM.01824-20. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

