Matching-adjusted indirect treatment comparison of chimeric antigen receptor T-cell therapies for third-line or later treatment of relapsed or refractory large B-cell lymphoma: lisocabtagene maraleucel versus tisagenlecleucel
- PMID: 35337365
- PMCID: PMC8953336
- DOI: 10.1186/s40164-022-00268-z
Matching-adjusted indirect treatment comparison of chimeric antigen receptor T-cell therapies for third-line or later treatment of relapsed or refractory large B-cell lymphoma: lisocabtagene maraleucel versus tisagenlecleucel
Abstract
Background: There are no head-to-head clinical studies comparing chimeric antigen receptor (CAR) T-cell therapies for the treatment of relapsed or refractory aggressive large B-cell lymphomas. Naive, indirect comparisons may be inappropriate, as the study designs and patient populations could differ substantially. Matching-adjusted indirect comparisons (MAIC) can reduce many biases associated with indirect comparisons between studies. To determine the comparative efficacy and safety of lisocabtagene maraleucel (liso-cel) to tisagenlecleucel, we describe an unanchored MAIC of the pivotal studies TRANSCEND NHL 001 (TRANSCEND; NCT02631044; liso-cel) and JULIET (NCT02445248; tisagenlecleucel).
Methods: Individual patient data (IPD) from TRANSCEND were available to the authors; for the JULIET pivotal study, summary-level data from the published study were used. To balance the populations between two studies, IPD from TRANSCEND were adjusted to match the marginal distribution (e.g., mean, variance) of clinical factors among patients from JULIET.
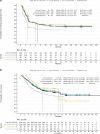
Results: Results from the primary MAIC showed liso-cel had statistically significant greater efficacy than tisagenlecleucel (objective response rate: odds ratio [OR] = 2.78, 95% confidence interval [CI]: 1.63‒4.74; complete response rate: OR = 2.01, 95% CI: 1.22‒3.30; progression-free survival: hazard ratio [HR] = 0.65, 95% CI: 0.47‒0.91; overall survival: HR = 0.67, 95% CI: 0.47‒0.95). MAIC of safety outcomes showed lower ORs for all-grade and grade ≥ 3 cytokine release syndrome, and grade ≥ 3 prolonged cytopenia for liso-cel when compared with tisagenlecleucel; there were no statistically significant differences detected for other safety outcomes.
Conclusions: Overall, this MAIC of two CAR T-cell therapies indicates liso-cel had favorable efficacy and a comparable or better safety profile relative to tisagenlecleucel.
Clinical trial registration: ClinicalTrials.gov identifiers: NCT02631044 and NCT02445248.
Keywords: CAR T-cell therapy; Indirect treatment comparison; Lisocabtagene maraleucel; Matching-adjusted indirect comparison; Tisagenlecleucel.
© 2022. The Author(s).
Conflict of interest statement
Guillaume Cartron has received consultancy fees from Celgene, a Bristol-Myers Squibb Company, and F. Hoffmann-La Roche; and honoraria from AbbVie, Celgene, a Bristol-Myers Squibb Company, F. Hoffmann-La Roche, Gilead Sciences, Janssen, and Sanofi. Christopher P. Fox has received honoraria from AbbVie, Adienne, AstraZeneca, Atara Biotherapeutics, Celgene, a Bristol-Myers Squibb Company, Genmab, Gilead Sciences, Incyte, Roche, Sunesis Pharmaceuticals, and Takeda; and grants for research from AbbVie, Adienne, Gilead Sciences, Roche, and Takeda. Fei Fei Liu, Ana Kostic, and Daniel Li are employees of Bristol Myers Squibb and hold stock in Bristol Myers Squibb. Jens Hasskarl was an employee of Celgene, a Bristol-Myers Squibb Company, at the time of this analysis and may hold stock in Bristol Myers Squibb. Ashley Bonner and Yixie Zhang are employees of EVERSANA, which received funding from Bristol Myers Squibb to conduct the analyses. David G. Maloney reports scientific advisory board membership for A2 Biotherapeutics for which he receives consultancy fees; equity holdings in A2 Biotherapeutics for which he has stock options; honoraria from Amgen, BioLineRx, Bristol Myers Squibb, Celgene, a Bristol-Myers Squibb Company, Genentech, Gilead Sciences, Janssen, Juno Therapeutics, a Bristol-Myers Squibb Company, Kite Pharma, a Gilead Company, Legend Biotech, MorphoSys, Novartis, and Pharmacyclics; intellectual property patents with Juno, a Bristol-Myers Squibb Company (not licensed, no royalties); and research funding paid directly to his institution from Celgene, a Bristol-Myers Squibb Company, Juno Therapeutics, a Bristol-Myers Squibb Company, and Kite Pharma, a Gilead Company. John Kuruvilla reports consultancy from AbbVie, Bristol Myers Squibb, Gilead, Karyopharm, Merck, Roche, and Seattle Genetics; honoraria from Amgen, Antengene, AstraZeneca, Celgene, a Bristol-Myers Squibb Company, Gilead, Janssen, Karyopharm, Merck, Novartis, Pfizer, Roche, Seattle Genetics, and TG Therapeutics; and research funding from AstraZeneca, Janssen, and Roche.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical