Closed-loop optimization of transcranial magnetic stimulation with electroencephalography feedback
- PMID: 35337598
- PMCID: PMC8940636
- DOI: 10.1016/j.brs.2022.01.016
Closed-loop optimization of transcranial magnetic stimulation with electroencephalography feedback
Abstract
Background: Transcranial magnetic stimulation (TMS) is widely used in brain research and treatment of various brain dysfunctions. However, the optimal way to target stimulation and administer TMS therapies, for example, where and in which electric field direction the stimuli should be given, is yet to be determined.
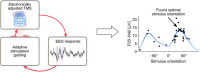
Objective: To develop an automated closed-loop system for adjusting TMS parameters (in this work, the stimulus orientation) online based on TMS-evoked brain activity measured with electroencephalography (EEG).
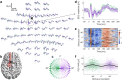
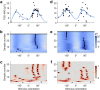
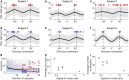
Methods: We developed an automated closed-loop TMS-EEG set-up. In this set-up, the stimulus parameters are electronically adjusted with multi-locus TMS. As a proof of concept, we developed an algorithm that automatically optimizes the stimulation orientation based on single-trial EEG responses. We applied the algorithm to determine the electric field orientation that maximizes the amplitude of the TMS-EEG responses. The validation of the algorithm was performed with six healthy volunteers, repeating the search twenty times for each subject.
Results: The validation demonstrated that the closed-loop control worked as desired despite the large variation in the single-trial EEG responses. We were often able to get close to the orientation that maximizes the EEG amplitude with only a few tens of pulses.
Conclusion: Optimizing stimulation with EEG feedback in a closed-loop manner is feasible and enables effective coupling to brain activity.
Keywords: Bayesian optimization; Closed-loop; Electroencephalography; Multi-channel TMS; Multi-locus TMS; Transcranial magnetic stimulation.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: P.L. has received consulting fees (unrelated to this work) from Nexstim Plc. R.J.I. is an advisor and a minority shareholder of Nexstim Plc. The other authors declare no competing interests.
Figures

Similar articles
-
Closed-loop TMS-EEG reactivity with occipital alpha-phase synchronized.J Neural Eng. 2022 Oct 7;19(5). doi: 10.1088/1741-2552/ac9432. J Neural Eng. 2022. PMID: 36137522
-
Automated search of stimulation targets with closed-loop transcranial magnetic stimulation.Neuroimage. 2020 Oct 15;220:117082. doi: 10.1016/j.neuroimage.2020.117082. Epub 2020 Jun 25. Neuroimage. 2020. PMID: 32593801
-
Delay Analysis in Closed-Loop EEG Phase-Triggered Transcranial Magnetic Stimulation.Annu Int Conf IEEE Eng Med Biol Soc. 2023 Jul;2023:1-4. doi: 10.1109/EMBC40787.2023.10340744. Annu Int Conf IEEE Eng Med Biol Soc. 2023. PMID: 38083335
-
Transcranial Magnetic Stimulation-Electroencephalography for Biomarker Discovery in Psychiatry.Biol Psychiatry. 2024 Mar 15;95(6):564-580. doi: 10.1016/j.biopsych.2023.12.018. Epub 2023 Dec 22. Biol Psychiatry. 2024. PMID: 38142721 Review.
-
Toward the establishment of neurophysiological indicators for neuropsychiatric disorders using transcranial magnetic stimulation-evoked potentials: A systematic review.Psychiatry Clin Neurosci. 2020 Jan;74(1):12-34. doi: 10.1111/pcn.12936. Epub 2019 Nov 2. Psychiatry Clin Neurosci. 2020. PMID: 31587446
Cited by
-
Advancements in Transcranial Magnetic Stimulation Research and the Path to Precision.Neuropsychiatr Dis Treat. 2023 Aug 23;19:1841-1851. doi: 10.2147/NDT.S414782. eCollection 2023. Neuropsychiatr Dis Treat. 2023. PMID: 37641588 Free PMC article. Review.
-
DELMEP: a deep learning algorithm for automated annotation of motor evoked potential latencies.Sci Rep. 2023 May 22;13(1):8225. doi: 10.1038/s41598-023-34801-9. Sci Rep. 2023. PMID: 37217502 Free PMC article.
-
A comparative study to assess synchronisation methods for combined simultaneous EEG and TMS acquisition.Sci Rep. 2025 Apr 14;15(1):12816. doi: 10.1038/s41598-025-97225-7. Sci Rep. 2025. PMID: 40229433 Free PMC article.
-
From dawn till dusk: Time-adaptive bayesian optimization for neurostimulation.PLoS Comput Biol. 2023 Dec 13;19(12):e1011674. doi: 10.1371/journal.pcbi.1011674. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38091368 Free PMC article.
-
A wearable repetitive transcranial magnetic stimulation device.Nat Commun. 2025 Mar 19;16(1):2731. doi: 10.1038/s41467-025-58095-9. Nat Commun. 2025. PMID: 40108144 Free PMC article.
References
-
- Lefaucheur J.-P., Aleman A., Baeken C., Benninger D.H., Brunelin J., Di Lazzaro V., et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018) Clin Neurophysiol. 2020;131:474–528. doi: 10.1016/j.clinph.2019.11.002. - DOI - PubMed
-
- Rossi S., Antal A., Bestmann S., Bikson M., Brewer C., Brockmöller J., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. 2021;132:269–306. doi: 10.1016/j.clinph.2020.10.003. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

