Implementation of corticosteroids in treatment of COVID-19 in the ISARIC WHO Clinical Characterisation Protocol UK: prospective, cohort study
- PMID: 35337642
- PMCID: PMC8940185
- DOI: 10.1016/S2589-7500(22)00018-8
Implementation of corticosteroids in treatment of COVID-19 in the ISARIC WHO Clinical Characterisation Protocol UK: prospective, cohort study
Abstract
Background: Dexamethasone was the first intervention proven to reduce mortality in patients with COVID-19 being treated in hospital. We aimed to evaluate the adoption of corticosteroids in the treatment of COVID-19 in the UK after the RECOVERY trial publication on June 16, 2020, and to identify discrepancies in care.
Methods: We did an audit of clinical implementation of corticosteroids in a prospective, observational, cohort study in 237 UK acute care hospitals between March 16, 2020, and April 14, 2021, restricted to patients aged 18 years or older with proven or high likelihood of COVID-19, who received supplementary oxygen. The primary outcome was administration of dexamethasone, prednisolone, hydrocortisone, or methylprednisolone. This study is registered with ISRCTN, ISRCTN66726260.
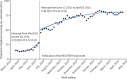
Findings: Between June 17, 2020, and April 14, 2021, 47 795 (75·2%) of 63 525 of patients on supplementary oxygen received corticosteroids, higher among patients requiring critical care than in those who received ward care (11 185 [86·6%] of 12 909 vs 36 415 [72·4%] of 50 278). Patients 50 years or older were significantly less likely to receive corticosteroids than those younger than 50 years (adjusted odds ratio 0·79 [95% CI 0·70-0·89], p=0·0001, for 70-79 years; 0·52 [0·46-0·58], p<0·0001, for >80 years), independent of patient demographics and illness severity. 84 (54·2%) of 155 pregnant women received corticosteroids. Rates of corticosteroid administration increased from 27·5% in the week before June 16, 2020, to 75-80% in January, 2021.
Interpretation: Implementation of corticosteroids into clinical practice in the UK for patients with COVID-19 has been successful, but not universal. Patients older than 70 years, independent of illness severity, chronic neurological disease, and dementia, were less likely to receive corticosteroids than those who were younger, as were pregnant women. This could reflect appropriate clinical decision making, but the possibility of inequitable access to life-saving care should be considered.
Funding: UK National Institute for Health Research and UK Medical Research Council.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests ABD reports grants from the UK Department of Health and Social Care (DHSC) during the conduct of the study, and grants from the Wellcome Trust outside the submitted work. JSN-V-T reports grants from the DHSC during the conduct of the study, and is seconded to the DHSC. PJMO reports personal fees from consultancies and from the European Respiratory Society; grants from the UK Medical Research Council (MRC), the MRC Global Challenge Research Fund, the EU, the NIHR Biomedical Research Centre, MRC–GSK, the Wellcome Trust, and the NIHR (Health Protection Research Unit in Respiratory Infections at Imperial College London); and is an NIHR senior investigator outside the submitted work; his role as President of the British Society for Immunology was unpaid but travel and accommodation at some meetings was provided by the society. JKB reports grants from the MRC. MGS reports grants from DHSC, NIHR UK, MRC UK, HPRU in Emerging and Zoonotic Infections, and University of Liverpool, during the conduct of the study; and is chair of the Infectious Diseases Science Advisory Board and minority shareholder of Integrum Scientific, Greensboro NC, and Independent external and non-remunerated member of Pfizer's External Data Monitoring Committee for their mRNA vaccine program(s) outside the submitted work. All other authors declare no competing interests.
Figures


References
-
- WHO Living guidance for clinical management of COVID-19. Nov 23, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1
-
- WHO Corticosteroids for COVID-19. Sept, 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-20...
-
- Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial Low-cost dexamethasone reduces death by up to one third in hospitalised patients with severe respiratory complications of COVID-19. June 16, 2020. https://www.recoverytrial.net/news/low-cost-dexamethasone-reduces-death-...
-
- Whitty C. Dexamethasone in the treatment of COVID-19: implementation and management of supply for treatment in hospitals. June 16, 2020. https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

