Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors
- PMID: 35338084
- PMCID: PMC8961117
- DOI: 10.1136/jitc-2021-003255
Phase I, multicenter, open-label study of intravenous VCN-01 oncolytic adenovirus with or without nab-paclitaxel plus gemcitabine in patients with advanced solid tumors
Abstract
Background: VCN-01 is an oncolytic adenovirus (Ad5 based) designed to replicate in cancer cells with dysfunctional RB1 pathway, express hyaluronidase to enhance virus intratumoral spread and facilitate chemotherapy and immune cells extravasation into the tumor. This phase I clinical trial was aimed to find the maximum tolerated dose/recommended phase II dose (RP2D) and dose-limiting toxicity (DLT) of the intravenous delivery of the replication-competent VCN-01 adenovirus in patients with advanced cancer.
Methods: Part I: patients with advanced refractory solid tumors received one single dose of VCN-01. Parts II and III: patients with pancreatic adenocarcinoma received VCN-01 (only in cycle 1) and nab-paclitaxel plus gemcitabine (VCN-concurrent on day 1 in Part II, and 7 days before chemotherapy in Part III). Patients were required to have anti-Ad5 neutralizing antibody (NAbs) titers lower than 1/350 dilution. Pharmacokinetic and pharmacodynamic analyses were performed.
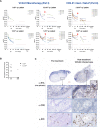
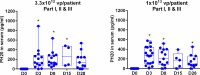
Results: 26% of the patients initially screened were excluded based on high NAbs levels. Sixteen and 12 patients were enrolled in Part I and II, respectively: RP2D were 1×1013 viral particles (vp)/patient (Part I), and 3.3×1012 vp/patient (Part II). Fourteen patients were included in Part III: there were no DLTs and the RP2D was 1×1013 vp/patient. Observed DLTs were grade 4 aspartate aminotransferase increase in one patient (Part I, 1×1013 vp), grade 4 febrile neutropenia in one patient and grade 5 thrombocytopenia plus enterocolitis in another patient (Part II, 1×1013 vp). In patients with pancreatic adenocarcinoma overall response rate were 50% (Part II) and 50% (Part III). VCN-01 viral genomes were detected in tumor tissue in five out of six biopsies (day 8). A second viral plasmatic peak and increased hyaluronidase serum levels suggested replication after intravenous injection in all patients. Increased levels of immune biomarkers (interferon-γ, soluble lymphocyte activation gene-3, interleukin (IL)-6, IL-10) were found after VCN-01 administration.
Conclusions: Treatment with VCN-01 is feasible and has an acceptable safety. Encouraging biological and clinical activity was observed when administered in combination with nab-paclitaxel plus gemcitabine to patients with pancreatic adenocarcinoma.
Trial registration number: NCT02045602.
Keywords: clinical trials as topic; gastrointestinal neoplasms; oncolytic virotherapy; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MB-P, MF-S, AM-B, MVM, EB, CB and MC are employees, and RS is consultant for VCN Biosciences. MC and RAle are co-inventors of one patent application concerning the expression of hyaluronidase by oncolytic adenoviruses and both have ownership interest in VCN Biosciences. RG-C has provided scientific advice and/or received honoraria or funding for continuous medical education from AAA, Advanz Pharma, Amgen, Bayer, BMS, HMP, Ipsen, Merck, Midatech Pharma, MSD, Novartis, PharmaMar, Pfizer, Pierre Fabre, Roche, Servier and Sanofi, and has received research support from Pfizer, BMS and MSD.
Figures

References
-
- Xia Z-J, Chang J-H, Zhang L, et al. . [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus]. Ai Zheng 2004;23:1666–70. - PubMed
-
- Di Paolo NC, Shayakhmetov DM. Adenovirus de-targeting from the liver. Curr Opin Mol Ther 2009;11:523–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
