Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA
- PMID: 35338333
- PMCID: PMC9433379
- DOI: 10.1038/s41418-022-00970-9
Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA
Abstract
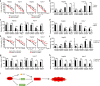
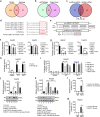
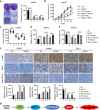
Ferroptosis, a novel form of regulated cell death induced by iron-dependent lipid peroxidation, plays an essential role in the development and drug resistance of tumors. Long noncoding RNA (lncRNA) nuclear paraspeckle assembly transcript 1 (NEAT1) has been reported to be involved in the regulation of cell cycle, proliferation, apoptosis, and migration of tumor cells. However, the function and molecular mechanism of NEAT1 in regulating ferroptosis in tumors remain unclear. Here, we found that ferroptosis inducers erastin and RSL3 increased NEAT1 expression by promoting the binding of p53 to the NEAT1 promoter. Induced NEAT1 promoted the expression of MIOX by competitively binding to miR-362-3p. MIOX increased ROS production and decreased the intracellular levels of NADPH and GSH, resulting in enhanced erastin- and RSL3-induced ferroptosis. Importantly, overexpression of NEAT1 increased the anti-tumor activity of erastin and RSL3 by enhancing ferroptosis both in vitro and in vivo. Collectively, these data suggest that NEAT1 plays a novel and indispensable role in ferroptosis by regulating miR-362-3p and MIOX. Considering the clinical findings that HCC patients are insensitive to chemotherapy and immunotherapy, ferroptosis induction may be a promising therapeutic strategy for HCC patients with high NEAT1 expression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

